The Science of Rheumatoid Arthritis Flashcards
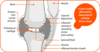
what does a normal joint look like?
2 bone ends
Covered by tissue that has no vascular and neuro called articular cartilage, made of one cell type the chondrocyte
Synovial fluid filled cavity made by the synovial membrane covering the joint capsule

what does the normal synovium look like?
Key tissue
Thin membrane covering inner tissue of joint
2 layers
Thin layer of lining consisting of fibroblasts and macrophages, just 2 types
Underneath there is the subintimal tissue
Lubrication happens as the synovium produces 2 main lubricants
articular cartilage is devoid of blood vessels or nerve fibres

what are the functions of the synovium?
maintenance of intact tissue surface
lubrication of cartilage
control of synovial fluid volume and composition (hyaluronan, lubricin)
nutrition of chondrocytes within joints
what does a rheumatoid joint look like?
In RA there is expansion of the synovium
Causes expansion of tissue
Destruction of cartilage and bone can be seen on x- ray, joint space narrowing
what is the definiton of RA?
- Rheumatoid arthritis is a chronic symmetric polyarticular inflammatory joint disease, which primarily affects the small joints of the hands and feet
- The rheumatoid synovitis (pannus) is characterised by inflammatory cell infiltration, synoviocyte proliferation and neoangiogenesis
- The synovial fluid in the joint cavity contains neutrophils, particularly during acute flares of RA
- The synovial pannus causes bone and cartilage destruction (deformities)
how is autoimmunity involved?
- Evidence of autoimmunity can be present in RA many years before the onset of clinical arthritis
- Autoantibodies, such as RFs and anti-citrullinated protein antibodies, are commonly associated with RA
- Autoantibodies occur in RA that recognise either joint antigens, such as type II collagen, or systemic antigens, such as glucose phosphate isomerase
- The autoantibodies potentially can contribute to inflammation through several mechanisms, including activation of complement
Autoantibody production - what is the difference between Seropositive and seronegative rheumatoid arthritis
Seropositive:
- Rheumatoid factor
- Anti-citrullinated protein antibody (ACPA)
- Diagnostic anti-CCP assays recognise citrullinated self-proteins - α-enolase, keratin, fibrinogen, fibronectin, collagen, vimentin
- Patients with ACPA+ disease have a less favourable prognosis
Negative if autoantibodies are not detectable
Rheumatoid factor: an auto-antibody to self IgG Fc

Genes play a key role in susceptibility to RA and disease severity, such as what?
- Twins studies implicate genetic factors in RA - Concordance rates 15-30% among monozygotic (identic) twins and Concordance rates 5% among dizygotic twins
- Association with HLA-DRB1 locus (HLA-DR4 serotype)
- Alleles containing a common amino acid motif (QKRAA – shared epitope) in the HLA-DRB1 region confer susceptibility - Role in promoting autoimmunity (e.g. altered antigen presentation), Molecular mimicry (e.g. with microbial proteins)
- Other genetic associations including polymorphisms in PTPN22, CTLA4, c-REL etc. aggregate functionally with immune regulation
- Genetic associations in RA are complex and involve many genes
- Distinct genetic associations for ACPA-positive and ACPA-negative RA
what Environmental factors are involved in causing RA?
- Smoking and bronchial stress (exposure to silica)
- Infectious agents have been associated with RA -Viruses (EBV, CMV), E. Coli, Mycoplasma, Periodontal disease (Porphyromonas gingivalis), Microbiome (gut microbes)
- Repeated insults in a genetically susceptible individual would lead to - Formation of immune complexes and rheumatoid factor (high-affinity autoAb against the Fc portion of Ig), Altered citrullination of proteins and breakdown of tolerance, with resulting ACPA response
what is the ACPA response in RA?
The most specific autoimmunity known for rheumatoid arthritis (RA) is reflected by generation of anti-citrullinated protein antibodies (ACPA). Presence of ACPA in established RA is associated with disease severity, while generation of ACPA at early developmental phases of RA can have a strong predictive value for progressing to the full-blown disease. Hence, development of ACPA may be of crucial importance to the pathogenesis of RA

what is the process of Development of Rheumatoid Arthritis?
Interplay between genetic and environmental factors that can lead to autoimmunity with immune mediated synovitis leading to arthritis and bone and cartilage destruction

how does Synovitis happen in RA
- Intimal lining hyperplasia and sublining infiltration (migration) with mononuclear cells, especially CD4 + T cells, macrophages, and B cells
- Lining FLS proliferate, become activated and “aggressive”
- Macrophages in the lining are activated
- Lymphocytes can either diffusely infiltrate the sublining or form lymphoid aggregates with germinal centres
- Sublining CD4+ T cells mainly display the memory cell phenotype
- Synovial B cells and plasma cells exhibit evidence of antigen-driven maturation and antibody production
- DCs can present antigens to T cells in synovial germinal centres
- Neoangiogenesis is induced by local hypoxic conditions and cytokines
- Insufficient lymphangiogenesis limits cellular egress
- Neutrophils are present in synovial fluid
Pathogenesis of Rheumatoid Arthritis
Synovitis = inflammation of the synovium
what effects odes this cause?
Villous hyperplasia
Infiltration of T cells, B cells, macrophages and plasma cells
Intimal cell proliferation (fibroblasts)
Production of cytokines and proteases
Increased vascularity
Self-amplifying process

how are T cells and cytokines involved?
- T-cell depleting strategies have limited efficacy
- Abatacept (fusion protein CTLA4-IgG1 Fc that blocks T-cell costimulation) is efficacious in RA
- Relatively low levels of T cell cytokines are present in RA synovium
- Shift from homeostasis to inflammation
- T-cell cytokines, such as IFN- γ and IL-17, are produced by Th1 cells or Th17 cells
- Regulatory T cell function, which suppresses activation of other T cells, is reduced
- T-cell-mediated B-cell activation
- Direct cell-cell contact with macrophages
how are B-cells, cytokines and autoantibodies involved?
- Synovial B cells are mainly localised in T-cell-B-cell-aggregates - Ectopic lymphoid follicles
- Pathogenic role for CD20+ B cells is confirmed by the efficacy of rituximab
- Plasma cells are widely distributed and are not targeted by anti-CD20 antibodies
- Role of B cells goes beyond production of autoAb
- Autoantigen presentation
- Cytokines (IL-6, TNF-α)
what are stromal cell cytokines?
- Macrophage and fibroblast cytokines are abundant in RA synovium
- Macrophages (M1) are activated by TLRs and NLRs
- Cytokine networks including TNF-α, IL-6, IL-1, IL-15, IL-18, IL-23 etc. perpetuate synovial inflammation
- Chemokines that recruit inflammatory cells into the joint generally are produced by macrophages and fibroblasts
- Anti-inflammatory cytokines such as IL-10 are produced in rheumatoid synovium in amounts insufficient to offset proinflammatory cytokines
what ar ethe effects of Inflammatory cytokines?
- Induce expression of endothelial-cell adhesion molecules
- Activate synovial fibroblasts, chondrocytes, osteoclasts
- Promote angiogenesis
- Suppress T-regs
- Activate leukocytes
- Promote autoAb production
- IL-6 mediates systemic effects - Acute-phase response, Anaemia, Cognitive dysfunction, Lipid metabolism dysregulation
how does Neoangiogenesis and what is its effects?
- Neo-angiogenesis provides nutrients to the hyperplastic synovium
- Hypoxic conditions and angiogenic factors such as IL-8 and VEGF enhance blood vessel proliferation in the synovium
- Microvascular endothelia in the synovium express adhesion molecules that guide circulating cells into the joint under the influence of chemoattractants
how does Cartilage and bone destruction
(Joint space narrowing and erosions) occur?
- Distinct mechanisms and cell types regulate cartilage degradation and bone destruction in RA
- Several classes of proteases, including metalloproteinases and aggrecanases are produced by FLS in the intimal lining layer
- Synovial lining cells, especially FLS, can attach to and invade cartilage in RA
- Bone destruction is mediated by osteoclasts that are activated under the influence of RANKL produced by RA synovium
Fibroblasts responsible for cartilage destruction and bone destruction is done by increased number of osteoclasts
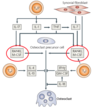
Cell types and cytokines in chronic inflammatory arthritis
what are some key cytokines?
- TNF – alpha
- IL – 1
- IL – 6
- RANK – ligand (OPGL)
- IL - 17
Diagram showing Factors that regulate osteoclast differentiation in RA
What are Systemic consequences of RA?
- Vasculitis, nodules, scleritis, amyloidosis = secondary to uncontrolled chronic inflammation
- Cardiovascular disease - Altered lipid metabolism, Elevated acute-phase reactants, Increased endothelial activation
- Fatigue and reduced cognitive function (secondary fibromyalgia) - Dysregulation of the HPA axis
- Liver - Elevated acute-phase response, Anaemia of chronic disease (IL-6 increases hepatocyte production of hepcidin, an iron-regulatory hormone)
- Lungs (interstitial lung disease, fibrosis)
- Muscles (sarcopoenia)
- Bone (osteoporosis)
- Secondary Sjogren’s syndrome

what are some unresolved questions and challenges?
- Which factors cause “loss of tolerance”
- Why inflammation is localised to the joint
- Can we promote immunologic resolution and restore immune homeostasis
- Why targeting the immune system may not be sufficient
- Can we repair damaged joints
Key points:
- Rheumatoid arthritis (RA) is a complex disease involving numerous cell types, including macrophages, T cells, B cells, fibroblasts, chondrocytes, and dendritic cells
- Several genes are implicated in susceptibility to RA and severity of disease, including class II major histocompatibility complex genes and PTPN22
- Repeated insults (smoking, infectious agents) in genetically susceptible individuals would lead to loss of tolerance and autoimmunity
- Evidence of autoimmunity, including high serum levels of autoAb (RFs and anti-CCP), can be present for many years before the onset of clinical arthritis
- Adaptive and innate immune responses in the synovium have been implicated in the pathogenesis
- Cytokine networks involving TNF-α, IL-6, and many other factors participate in disease perpetuation (therapeutic targets)
- Bone and cartilage destruction are primarily mediated by osteoclasts and fibroblast-like synoviocytes


