MSK - Biochemistry - Glycolysis; Gluconeogenesis; Glycogen Metabolism Flashcards
How many reactions occur in glycolysis?
What phase are the first 5 known as?
What phase are the second 5 known as?
10;
the investment/preparatory phase;
the payoff phase
What are the two goals of the investing/preparatory phase of glycolysis?
How many ATP are invested at this point?
- To make the process irreversible
- To split the 6-carbon sugar into two 3-carbon sugars
2
By the end of the glycolytic investing/preparatory phase, glucose has been split into two molecules known as:
Glyceraldehyde-3-phosphate
In the second five glycolytic reactions, the payoff phase, what high energy molecules are produced?
What is the net gain of glycolysis?
4 ATP, 2 NADH;
2 ATP, 2 NADH
Of the ten glycolytic reactions, which are the most important for a healthcare provider to know?
1, 3, 6, 7, (9), 10
The GLUT transporters work via what type of transport?
Facilitated diffusion
What GLUT transporters are found in virtually all mammalian tissues and maintain basal glucose levels?
GLUT1, GLUT3
What GLUT transporter is found in liver and pancreatic cells (as well as the basolateral portion of epithelium in the small intestine) and removes excess glucose from the blood / controls insulin secretion?
GLUT2
What GLUT transporter is found in muscle and fat cells and is insulin sensitive?
GLUT4
What GLUT transporter is found in mucosal membranes and spermatozoa for fructose transport?
GLUT5
GLUT1 and GLUT3 are found in what tissues?
What is their purpose?
Virtually all mammalian tissues - maintain cellular basal glucose needs
GLUT2 is found in what tissues?
What is its purpose?
Pancreatic tissues - control insulin secretion;
Liver and small intestinal tissues - removes excess glucose from blood
GLUT4 is found in what tissue(s)?
It is ________-sensitive.
Muscle and adipose;
insulin
GLUT5 transporters move what substance?
Fructose
For GLUT1, GLUT2, GLUT3, GLUT4, and GLUT5, state the relative Km for each.
(place them in order of increasing Km)
GLUT3 - 1 mM
GLUT1 - 1 - 2 mM
GLUT4 - 5 mM
GLUT5 - 10 mM
GLUT2 - 15 - 20 mM
Normal physiological glucose levels are around: ____mM.
5.5
(100 mg/dl)
Which GLUT transporters are transporting glucose almost all the time?
Which GLUT transporters are transporting glucose in the well-fed state only?
GLUT1, GLUT3, GLUT4;
GLUT2
(note: GLUT5 transports fructose)
Describe the basic steps of insulin secretion.
1. Glucose levels rise (GLUT2 Km = 15 - 20 mM)
2. GLUT2 transporters allow glucose into pancreatic β cells
3. ATP is formed via glycolysis
4. ATP turns off K+ leak channels
5. The cell depolarizes
6. Calcium floods the cell and insulin is released

What is the 3-carbon end product of glycolysis?
Pyruvate
How do anti-hyperglycemic drugs such as sulfonylureas or meglitinides work?
What drug does the opposite?
They close ATP-sensitive K+ channels in pancreatic β cells
(note: these are channels that were pumping K+ into the cell);
diazoxide (treats insulinoma-induced hypoglycemia)

Should type I diabetics be given sulfonylureas and/or meglitinides?
Should type II diabetics be given sulfonylureas and/or meglitinides?
They shouldn’t. They don’t have β cells;
Yes
Where are GLUT4 transporters when glucose levels are low?
Where are GLUT4 transporters when glucose levels are high?
Endocytosed;
on the plasma membrane (exocytosed)
What is the first step of glycolysis?
Why is this step important (3 reasons)?
Hexokinase adds a phosphate to glucose (making G6P).
- Energy investment
- Traps glucose in cell
- Ionic charges increase favorability of subsequent interactions
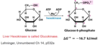
What are substrates and product of the first step of glycolysis/
Glucose (+ hexokinase + ATP + Mg2+) –> Glucose-6-phosphate

Why is the first step of glycolysis (shown below) a favorable reaction (- ΔG)?

ATP coupling
Why is the hexokinase found in the liver (hexokinase IV) called glucokinase?
Does it have any inhibitors?
It only acts on glucose;
yes, F6P
What is the [glucose] Km for hexokinase I (or II and III)?
What is the [glucose] Km for hexokinase IV (glucokinase)?
0.05 mM;
5 mM
Which is allosterically inhibited by G6P, hexokinase I or glucokinase?
Hexokinase I
When GLUT2 transports glucose into liver cells, what effect does this have on glucokinase?
It draws it out of the nucleus

Which of the following would inactive liver glucokinase by shuttling it into the nucleus, incoming glucose or liver gluconeogenesis?
Liver gluconeogenesis

What happens in step two of glycolysis?
What enzyme is responsible?
G6P is turned into F6P;
phosphohexose isomerase
What is the commitment step of glycolysis?
Via what enzyme?
Step 3;
phosphfructokinase-1 (PFK-1)

What are the two investment steps of the preparatory phase (1st five steps) of glycolysis?
Steps 1 and 3
What are the substrates and main product of the 3rd step of glycolysis?
F6P (+ PFK-1 + ATP + Mg2+) –> F1,6BP

What are some allosteric activators of the third step of glycolysis (shown below)?

AMP, ADP, F2,6BP

What are some allosteric inhibitors of the third step of glycolysis (shown below)?

ATP, citrate

What effect does F2,6BP have on the third step of glycolysis (shown below)?
What effect does citrate have on the third step of glycolysis (shown below)?

Allosteric activation;
allosteric inhibition

What enzyme catalyzes the formation of F2,6BP?
What enzyme catalyzes the breakdown of F2,6BP?
PFK-2;
F2,6BPase -2

True/False.
PFK-2 and F2,6BPase-2 are two separate domains of the same enzyme and both can be active at the same time.
False.
They are two separate domains of the same enzyme, but only one is active at any one point in time
What effect does glucagon have on FBPase-2 activity?
How?
Increased;
phosphorylation of the enzyme via PKA
What effect does insulin have on PFK-2 activity?
How?
Increased;
dephosphorylation of the enzyme via phosphoprotein phosphatase
Does glucagon activate the F2,6BPase-2 or PFK-2 domain?
How?
Does insulin activate the F2,6BPase-2 or PFK-2 domain?
How?
F2,6BPase-2, phosphorylation via PKA;
PFK-2, dephosphorylation via PPP
What effect does glucagon have on F2,6BP levels? How?
What effect does insulin have on F2,6BP levels? How?
Decreased, via PKA –> F2,6BPase-2 activity;
increased, via PPP –> PFK-2 activity
Does F2,6BP allosterically activate or inhibit glycolysis?
Activate
Describe glucagon’s overall effects on the following:
PKA or PPP
F2,6BPase-2 or PFK-2
F2,6BP
Glycolysis
Gluconeogenesis
PKA activity increased
F2,6BPase-2 activity increased
F2,6BP decreased
Glycolysis inhibited
Gluconeogenesis stimulated

Describe insulin’s overall effects on the following:
PKA or PPP
F2,6BPase-2 or PFK-2
F2,6BP
Glycolysis
Gluconeogenesis
PPP activity increased
PFK-2 activity increased
F2,6BP increased
Glycolysis stimulated
Gluconeogenesis inhibited

What happens in step 4 of glycolysis (starting with F1,6BP)?
What happens in step 5 of glycolysis?
The F1,6BP is chopped into DHAP and glyceraldehyde-3-phosphate;
the DHAP is isomerized into glyceraldehyde-3-phosphate
Name an allosteric inhibitor of hexokinase I.
G6P
What occurs in step 6 of glycolysis?
Why is this important?
Inorganic phosphate is added to glyceraldehyde-3-phosphate to form 1,3-bisphosphoglycerate;
there are now two phosphates on each molecule, yielding four ATP total in the payoff phase (2 ATP net)

In step 6 of glycolysis, inorganic phosphate is added to glyceraldehyde-3-phosphate to form 1,3-bisphosphoglycerate.
What enzyme accomplishes this and what electron acceptor is needed?
Glyceraldehyde-3-phosphate dehydrogenase;
NAD+
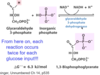
What steps of glycolysis involve ATP hydrolysis?
What steps of glycolysis involve NADH production?
What steps of glycolysis involve ATP production?
Steps 1 and 3;
step 6;
steps 7 and 10
Why is it important that F1,6BP (from an original glucose molecule) is cleaved into two separate halves during glycolysis?
The energy generated in the payoff phase is doubled
What is the term that describes the fact that reactions with fairly neutral ΔGs with occur rapidly if very thermodynamically stable reactions occur later in the flow?
(E.g. the less favorable steps 2 and 5 of glycolysis happen readily because steps 1, 3, and 10 occur?)
Substrate flux
What happens in step 7 of glycolysis?
Via what enzyme?
1,3-Bisphosphoglycerate is turned into 3-phosphoglycerate and ATP is formed;
phosphoglycerate kinase

The ATP production in glycolysis is termed ___________-_____ phosphorylation.
This in contrast with __________ phosphorylation.
Substrate, level;
oxidative
Which must have a higher energy state, 1,3-bisphosphoglycerate or ATP?
(reaction 7 of glycolysis shown below)
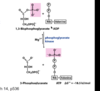
1,3-Bisphosphoglycerate
(used for substrate-level phosphorylation)
What happens in step 8 of glycolysis (starting with 3-phosphoglycerate)?
What happens in step 9 of glycolysis?
3-PG is isomerized to 2-PG by PG mutase;
2-PG is dehydrated to phosphoenolpyruvatase by enolase
What happens in step 10 of glycolysis?
Via what enzyme?
Phosphoenolpyruvate is turned into pyruvate and ATP;
(substrate-level phosphorylation)
pyruvate kinase

What glycolytic intermediate is one of the highest energy compounds that we know of?
Phosphoenolpyruvate
(much higher than ATP or 1,3-BPG)
Which must have a higher energy state, phosphoenolpyruvate or ATP?
(reaction 10 of glycolysis shown below)

Phosphoenolpyruvate
(used for substrate-level phosphorylation)
What are the three important products of glycolysis?
2 Pyruvate
2 NADH
2 ATP
What are the glucose-derived substrates and intermediates of glycolysis (starting at glucose and ending at pyruvate)?
Bold each one that is used for substrate-level phosphorylation to form ATP.
Place an asterisk next to each one that is used for glycolytic NADH production.
[1 Glucose] –>
G6P –>
F6P –>
F1,6BP –>
GAP* + DHAP (DHAP –> GAP*) –>
1,3-BPG –>
3-PG –>
2-PG –>
PEP –>
[2 Pyruvate]
In the liver, what hormone serves to inactivate pyruvate kinase?
Via what enzyme?
Glucagon;
PKA
What are some allosteric activators of pyruvate kinase?
What are some allosteric inhibitors of pyruvate kinase?
ADP, phosphoenolpyruvate, F1,6BP;
ATP, acetyl-CoA, long-chain fatty acids, alanine
What are some allosteric activators of pyruvate kinase?
ADP, phosphoenolpyruvate, F1,6BP
What are some allosteric inhibitors of pyruvate kinase?
ATP, acetyl-CoA, long-chain fatty acids, alanine
In the liver, what hormone serves to activate pyruvate kinase?
Via what enzyme?
Insulin;
PPP
During the 7th step of glycolysis (shown below), 1,3-BPG can be isomerized to become what substance?
What effect will this have on the blood?
What effect will this have had on glycolytic ATP production?

2,3-BPG;
causes oxygen release from hemoglobin;
one less ATP (because step 7 was skipped)

What are the two most common genetic causes of hemolytic anemia?
- G6PD deficiency
- Pyruvate kinase deficiency
What is the main sign of pyruvate kinase deficiency that is often first seen at high altitudes?
Why does it occur?
Hemolytic anemia;
step 7 of glycolysis is circumvented by 2,3BPG formation
(step 10 is already inhibited by the PK deficiency:
subtrate-level ATP synthesis is stopped, and that’s all erythrocytes have for energy production)
Why would an individual with COPD have a resultant acidosis?
(2 reasons)
Increase in carbonic acid due to trapped CO2
(respiratory acidosis)
Lack of O2 = pause in oxidative phosphorylation; switch to anerobic glycolysis for energy production –> lactic acid production
(lactic acidosis)
What is the purpose of anerobic acidosis?
To regenerate NAD+
by turning pyruvate into lactate

True/False.
A patient with severe COPD may present with simultaneous respiratory and metabolic acidosis.
True
(O2 deficiency / lactic acidosis –> metabolic cause;
CO2 buildup –> respiratory cause)
During the day (from about breakfast to midnight) blood sugar comes mostly from what two sources?
During the night (midnight to breakfast), it comes mostly from what source?
Diet, glycogenolysis;
gluconeogenesis
How many irreversible steps are there in glycolysis?
So how does gluconeogenesis get around this?
3;
with alternate enzymes (4 of them)

Why are the effects of hexokinase, PFK-1, and pyruvate kinase deemed ‘irreversible?’
They have such negative ΔGs and are hugely thermodynamically favorable
For the most part, gluconeogenesis is just glycolysis in the reverse direction.
How many unique gluconeogenic enzymes are there?
What tissues contain them?
Only 4;
hepatic, kidney, and intestinal epithelial tissues
What are normal glucose physiological levels?
(in both mg/dl and mM)
100 mg/dl;
5.5 mM
With key exceptions in three steps, gluconeogenesis is basically just ___________ in reverse.
Glycolysis

What are the four unique gluconeogenic enzymes?
Pyruvate carboxylase
Phosphoenolpyruvate carboxykinase
Fructose 1,6-bisphosphatase
Glucose 6-phosphatase

What gluconeogenic enzymes allow the body to skip backwards past the ‘irreversible’ step 10 of glycolysis (shown below)?

Pyruvate carboxylase
Phosphoenolpyruvate carboxykinase
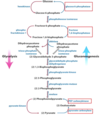
What gluconeogenic enzymes allow the body to skip backwards past the ‘irreversible’ step 3 of glycolysis?
Where is this enzyme found?
Fructose 1,6-bisphosphatase;
the cytosol
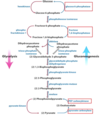
What gluconeogenic enzymes allow the body to skip backwards past the ‘irreversible’ step 1 of glycolysis?
Where is this enzyme found?
Glucose 6-phosphatase;
the ER

What are the first two steps of gluconeogenesis?
What step of glycolysis and enzyme has this allowed us to reverse?
1. Pyruvate carboxylase combines CO2 + biotin + ATP + pyruvate to make oxaloacetate
2. Phosphoenolpyruvate carboxykinase adds GTP to oxaloacetate to release that same CO2 and make phosphoenolpyruvate (PEP);
Step 10, pyruvate kinase
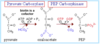
What step of gluconeogenesis occurs in the mitochondria and requires biotin as a cofactor?
Step 1
(Pyruvate carboxylase combines CO2 + biotin + ATP + pyruvate to make oxaloacetate)
Identify the cellular compartment where each of the following gluconeogenic enzymes are found:
Pyruvate carboxylase
Phosphoenolpyruvate carboxykinase
Fructose 1,6-bisphosphatase
Glucose 6-phosphatase
Mitochondria
Mitochondria, cytosol
Cytosol
Endoplasmic reticulum
What tissues have all of the following enzymes?
Pyruvate carboxylase
Phosphoenolpyruvate carboxykinase
Fructose 1,6-bisphosphatase
Glucose 6-phosphatase
Liver, kidney, intestinal epithelium
(Note: adipose tissue has PC and PEP-CK for glyceroneogenesis)
How can G6P access the ER to be acted upon by glucose 6-phosphatase in gluconeogensis?
G6P transporter 1 is found on the ER membrane
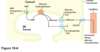
How many steps does it typically take to convert alanine to pyruvate?
1
What is an example of a substrate that can be turned into DHAP and then GAP, a gluconeogenic/glycolytic intermediate?
What is an example of a substrate that can be turned into oxaloacetate, a gluconeogenic intermediate?
What is an example of a substrate that can be turned into pyruvate, a gluconeogenic intermediate?
Glycerol;
some amino acids;
lactate, alanine
Most of the glucose made in gluconeogenesis is made from the starting substrate:
Alanine
What is the energy expense of gluconeogenesis to produce one glucose molecule?
4 ATP + 2 GTP + 2 NADH
What is the main allosteric activator of PFK-1 and allosteric inhibitor of F1,6BPase?
F2,6BP
What hormone does the pancreas release in the starved state?
What hormone does the pancreas release in the fed state?
Glucagon (islet α cells)
Insulin (islet β cells)
What effect does glucagon have on pyruvate kinase?
Inactivation
(through phosphorylation via PKA)
What effect does acetyl-CoA have on pyruvate carboxylase?
Activation
(for increased oxaloacetate to be available in gluconeogenesis or the TCA)
A high glucagon : insulin ratio stimulates the synthesis of what compounds?
A high insulin : glucagon ratio stimulates the synthesis of what compounds?
Pyruvate carboxykinase, F1,6BPase, G6Pase
(gluconeogenic enzymes)
glucokinase, PFK-1, pyruvate kinase
(main glycolytic enzymes)
Although adipose tissue cannot perform gluconeogenesis, it still has the first two enzymes of gluconeogenesis (pyruvate carboxylase and PEP carboxykinase).
Why is that?
They are used to synthesize glycerol 3-phosphate from pyruvate
(glyceroneogenesis)
How many fatty acids can be attached to one glycerol molecule?

Three
(to make a triglyceride)

What effect does glucagon have on serum fatty acid levels?
They increase dramatically
In which organs does glyceroneogenesis occur?
The liver and adipose tissue
In what tissues is glycogen synthesized and stored?
The liver and skeletal muscle
What enzyme is found in hepatic tissues but not skeletal tissues and explains why muscle does not break down glycogen to maintain blood sugar levels as the liver does?
Glucose 6-phosphatase
(the muscle cannot release its sugar as the liver can)
What bond is found in linear glycogen chains?
What bond is found in glycogen branch points?
α-1,4 glycosidic linkages
α-1,6 glycosidic linkages
Although glucose is quickly phosphorylated by hexokinase when it enters the muscle (creating glucose 6-phosphate), why is this not yet useful to glycogen synthesis?
So, what is the first step of glycogen synthesis?
Glycogen synthesis starts with glucose 1-phosphate;
G6P is converted to G1P by phosphoglucomutase
True/False.
The conversion of G6P to G1P by phosphoglucomutase is necessary to glycogen synthesis and thus irreversible.
False.
While it is true that this step is necessary for glycogenesis, the reaction is readily reversible.
What are the first two steps of glycogen synthesis?
(Let’s assume a glucose molecule has entered the myocyte through a GLUT4 transporter and just been phosphorylated to G6P by hexokinase I)
1. G6P –> G1P (via phosphoglucomutase)
2. G1P –> UDP - glucose (via UDP-glucose pyrophosphorylase*)
(Note*: 2 phosphate groups were hydrolyzed from UTP and UMP was attached to G1P to form UDP-glucose)
What are the first two enzymes of glycogenesis?
What is the third step?
Phosphoglucomutase;
UDP-glucose pyrophosphorylase;
UDP-glucose –> added to glycogen (α-1,4 glycosidic bond)
A deficiency of what enzyme causes type 0 glycogen storage disease? (state the alternate name if there is one)
Identify any majorly affected organs.
What are the main signs/symptoms?
Glycogen synthase;
the liver;
hypoglycemia, elevated ketones,
early death
A deficiency of what enzyme causes type I glycogen storage disease? (state the alternate name if there is one)
Identify any majorly affected organs.
What are the main signs/symptoms?
(von Gierke’s) glucose 6-phosphatase;
the liver;
hepatomegaly, kidney failure
True/False.
von Gierke’s disease affects skeletal muscle and hepatic tissues.
False.
Skeletal muscle lacks glucose 6-phosphatase;
type I glycogen storage disease affects the liver and kidney
A deficiency of what enzyme causes type II glycogen storage disease? (state the alternate name if there is one)
Identify any majorly affected organs.
What are the main signs/symptoms?
(Note: has infantile, juvenile, and adult presentations)
(Pompe’s) lyosomal glucosidase;
skeletal and cardiac muscle;
- infantile form* - death by age 2
- juvenile form* - muscle defects
- adult form* - as a muscular dystrophy
A deficiency of what enzyme causes type IIIa glycogen storage disease? (state the alternate name if there is one)
Identify any majorly affected organs.
What are the main signs/symptoms?
(Cori’s or Forbe’s) Debranching enzyme;
the liver, skeletal muscle, cardiac muscle;
myopathy, infantile hepatomegaly
Type IIIb glycogen storage disease is similar to type IIIa (Cori’s or Forbe’s) with what key exception?
It is a deficiency of debranching enzyme in the liver only
(presents only as infantile hepatomegaly)
A deficiency of what enzyme causes type IV glycogen storage disease? (state the alternate name if there is one)
Identify any majorly affected organs.
What are the main signs/symptoms?
(Anderson’s) Branching enzyme;
the liver, skeletal muscle;
hepatosplenomegaly, myoglobinuria
A deficiency of what enzyme causes type V glycogen storage disease? (state the alternate name if there is one)
Identify any majorly affected organs.
What are the main signs/symptoms?
(McArdle’s) Glycogen phosphorylase [in muscle];
skeletal muscle;
exercise-induced cramps, myoglobinuria
A deficiency of what enzyme causes type VI glycogen storage disease? (state the alternate name if there is one)
Identify any majorly affected organs.
What are the main signs/symptoms?
(Hers’s) glycogen phosphorylase [in the liver];
the liver;
hepatomegaly
What differentiates McArdle’s disease from Hers’s disease?
Location;
McArdle’s - glycogen phosphorylase in skeletal muscle is deficient
Hers’s - glycogen phosphorylase in the liver is deficient
A deficiency of what enzyme causes type VII glycogen storage disease? (state the alternate name if there is one)
Identify any majorly affected organs.
What are the main signs/symptoms?
(Tarui’s) PFK-1 [in muscle];
Muscle, erythrocytes;
Same as McArdle’s + hemolytic anemia
A deficiency of what enzyme causes type XI glycogen storage disease? (state the alternate name if there is one)
Identify any majorly affected organs.
What are the main signs/symptoms?
(Fanconi-Bickel) GLUT2;
liver;
failure to thrive, hepatomegaly, rickets, kidney dysfunction
Name the alternate name (if applicable) and missing enzyme for each of the following glycogen storage diseases:
Type 0
Type Ia
Type II
Glycogen synthase;
(von Gierke’s) glucose 6-phosphatase
(Pompe’s) lysosomal glucosidase
Name the alternate name (if applicable) and missing enzyme for each of the following glycogen storage diseases:
Type IIIa
Type IIIb
Type IV
(Cori’s or Forbe’s) Debranching enzyme
Liver debranching enzyme
(Anderson’s) Branching enzyme
Name the alternate name (if applicable) and missing enzyme for each of the following glycogen storage diseases:
Type V
Type VI
(McArdles’s) Muscle glycogen phosphorylase
(Hers’s) Liver glycogen phosphorylase
Name the alternate name (if applicable) and missing enzyme for each of the following glycogen storage diseases:
Type VII
Type XI
(Tarui’s) Muscle PFK-1
(Fanconi-Bickel) GLUT2
What is the techinical name for the branching enzyme of glycogenesis?
What type of bond does it form between UDP-glucoses?
Glycosyl 4,6 transferase;
α-1,6 glycosidic linkages
True/False. (for each of the following)
The branching of glycogen (A) increases storage space, (B) increases the number of ends and thus the speed with which it can be stored or broken down, and (C) decreases its solubility.
A. True
B. True
C. False; branching increases solubility
De novo glycogenesis needs a starting point and can’t just start as free-floating UDP-glucose molecules.
What is the primer protein that serves as this initialization point?
Glycogenin
Anderson’s disease affects what organ tissues?
(type IV glycogen storage disease)
hepatic tissue and skeletal muscle
What enzyme chops single G1P molecules from glycogen in a linear manner?
What is this enzyme’s cofactor?
Glycogen phosphorylase;
Pyridoxal phosphate (vitamin B6)
What are the four primary enzymes of glycogenesis?
In de novo glycogenesis, they build off what primer?
Phosphoglucomutase;
UDP-glucose pyrophosphorylase;
glycogen synthase;
Glycosyl 4,6 transferase (branching enzyme)
glycogenin
Describe the two steps of glycogen debranching during glycogenolysis.
Name the two enzymes involved
1. 3 G1P residues are transfered from the branch, leaving just one at the branch point (enzyme: glucosyl 4,4-transglycosidase)
- α-1,6 glucosidase cleaves the remaining G1P

Lysosomal acidic α-glucosidase is the equivalent of what other enzyme (but found in the lysosome instead)?
Similar to this other enzyme, it cleaves what type of bond?
Glycogen phosphorylase;
α-1,4 glycosidic linkages
Name all four enzymes of glycogenolysis that take branched glycogen and turn it into G6P molecules.
Glycogen phosphorylase;
*glucosyl 4,4 transglycosidase;
*α-1,6 Glucosidase;
phosphoglucomutase
(*both part of debranching enzyme)
What cofactor does glycogen phosphorylase require?
It is basically the active form of vitamin __.
Pyridoxal phosphate;
B6
Which of the following is activated by phosphorylation and which is deactivated by phosphorylation?
Glycogen synthase
Glycogen phosphorylase
Activated: glycogen phosphorylase
Inactivated: glycogen synthase
Via what covalent modification is glycogen synthase activated?
What hormone would likely result in this effect?
Dephosphorylation;
insulin (and PP1 –> inactivating glycogen synthase kinase 3)
Via what covalent modification is glycogen phosphorylase activated?
What hormone would likely result in this effect?
Phosphorylation;
glucagon/epinephrine (and PKA –> activating phosphorylase kinase)
What molecule, at high concentrations, will allosterically inactivate glycogen phosphorylase?
Glucose
Why do individuals with pyruvate kinase deficiency often manifest with hemolytic anemia at high altitudes?
(Hint: steps 7 and 10 of glycolysis are the only ATP-producing mechanisms that RBCs have)
As 1,3-BPG is isomerized to 2,3-BPG (because of the altitude), the afflicted individual loses their only remaining ATP-production step of glycolysis (step 7)
(step 10 is already compromised by the pyruvate kinase deficiency)
True/False.
Erythrocytes in patients with pyruvate kinase deficiency are getting all their ATP from only step 10 of glycolysis.
False.
The RBCs are getting all their ATP from only step 7
(step 10 is compromised by the PK deficiency)
How does 2-deoxyglucose inhibit ATP production and glycolysis?
It is phosphorylated by hexokinase and then builds up in the cell as 2-deoxyglucose 6-phosphate
(it cannot progress to the second step of glycolysis)
What glycolytic enzyme does iodoacetamide bind and inhibit?
Glyceraldehyde 3-phosphate dehydrogenase
What type of phosphorylation does arsenate (a phosphate analog) inhibit?
Substrate-level
What glycolytic enzyme does fluoride inhibit?
Enolase
Which is allosterically inhibited by F6P, hexokinase I or glucokinase?
Glucokinase
Which amino acid is the most common substrate for kinase modification of the proteins that control the rates of glucose catabolism and glycogen formation?
Serine
Which enzyme catalyzes a reaction that is most likely to be the committed step in the pathway?
E1 –> E2 –> E3 –> E4
E1 –> E2 –> E3 –> E4
The metabolite of which enzyme is most likely to be feedback inhibitor for the pathway?
E1 –> E2 –> E3 –> E4
E1 –> E2 –> E3 –> E4
What high-energy bond is broken to induce glycogen storage?
UTP
Most steps of glycogenolysis yield G1P monomers.
Which reaction yields unphosphorylated glucose?
Alpha-1,6 glucosidase
(domain of debranching enzyme)
What are the two domains of the debranching enzyme in glycogenolysis?
What is branching enzyme’s name?
Glucosyl 4,4-transglycosidase,
alpha-1,6 glucosidase;
glycosyl 4,6 transferase


