Cardio - Biochemistry - Pyruvate Dehydrogenase Complex; Citric Acid Cycle; Oxidative Phosphorylation Flashcards
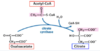
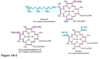
What are the major substrate (1) and products (3) of the pyruvate dehydrogenase complex?
Pyruvate —> acetyl-CoA, CO2, NADH
How many enzymatic subunits make up the pyruvate dehydrogenase complex?
What are they termed?
3;
E1, E2, E3

In what order do substrates encounter the enzymes of the pyruvate dehydrogenase complex?

E1, E2, E3
(moving inwards from the outer enzyme rings)

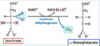
What are the five cofactors of the pyruvate dehydrogenase complex?
Thiamine (thiamine pyrophosphate) (B1);
riboflavin (B2);
niacin (B3);
pantothenic acid (B5);
lipoic acid
Which B-vitamins are represented in the cofactors of the pyruvate dehydrogenase complex?
B1, B2, B3, and B5
(thiamine pyrophosphate (thiamine), FAD (riboflavin), NAD+(niacin), coenzyme A (pantothenic acid))
What vitamins are associated with FAD and NAD+ formation, respectively?
Riboflavin, niacin
True/False.
The overall ΔG for the pyruvate dehydrogenase complex reaction is negative.
True.
(The reaction is spontaneous and thermodynamically favorable)
Describe the content of the mitochondrial intermembrane space.
Essentially contiguous with the cytosol
True/False.
The outer mitochondrial membrane provides a tight barrier to nearly all contents of the cytosol.
False;
porins allow free movement of most low-weight cytosolic components
What gives the outer mitochondrial membrane its permeability?
Porins
What structures allow pyruvate to enter the mitochondrial matrix?
Where are they found?
H+/pyruvate symporters;
the inner mitochondrial membrane (the outer is porous)
CO2 is produced in which step of the pyruvate dehydrogenase complex reaction?
(which enzyme?)
Step 1
(E1)

The first step of the pyruvate dehydrogenase complex involves the E_ enzyme. CO2 is released and the remaining 2-carbon substrate ends up bound to __________ __________.
1;
thiamine pyrophosphate

True/False.
The substrate of the pyruvate dehydrogenase complex is covalently bound to the enzymatic subunits during the reaction.
True.

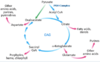
How many steps are there in the pyruvate dehydrogenase complex reaction?
5
There are 5 steps to the pyruvate dehydrogenase complex reaction.
How many of these reactions are focused on creating acetyl-CoA?
What is the purpose of the remaining reactions?
The first 3;
to reoxidize lipoyllysine for further reactions
What is the term for the enzymatic process used by the pyruvate dehydrogenase complex in which the substrate is (covalently) bound and passed from one enzyme to the next?
Substrate channeling

What high-energy electron carrier is produced in step 4 of the PDH complex?
To what other high-energy electron carrier does it transfer its electrons in step 5?
FADH2;
NADH

Thiamine pyrophosphate is involved in which two steps of the PDH complex?
The first two

Acetyl-CoA is generated in which step of the PDH complex?
What cofactor is involved?
Step 3;
pantothenic acid (vitamin B5)

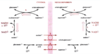
Match each of the following cofactors to the enzyme of the PDH complex with which they are associated:
Thiamine pyrophosphate (vitamin B1)
Riboflavin (FAD) (vitamin B2)
Niacin (NAD+) (vitamin B3)
Pantothenic acid (part of CoA) (vitamin B5)
Lipoic acid
Thiamine pyrophosphate E1
Riboflavin (FAD) E3
Niacin (NAD+) E3
Pantothenic acid (part of CoA) E2
Lipoic acid E2

Which cofactors of the PDH complex are associated with E1?
And E2?
And E3?

E1
Thiamine pyrophosphate
E2
Pantothenic acid (part of CoA)
Lipoic acid
E3
Riboflavin (FAD)
Niacin (NAD+)

Put the following cofactors in order of the enzymatic steps in which they participate in the 5 steps of the PDH complex:
Thiamine pyrophosphate
FAD
NAD+
CoA
Lipoic acid
Step 1 - Thiamine pyrophosphate
Step 2 - Thiamine pyrophosphate; lipoic acid
Step 3 - Lipoic acid; CoA
Step 4 - FAD
Step 5 - FAD; NAD+

Which enzymatic steps of the PDH complex (5) take place at E1?
At E2?
At E3?
1, 2
3
4, 5