Biochemistry 9: Carbohydrate Metabolism I Flashcards
GLUT 2
low-affinity transporter in hepatocytes and pancreatic cells
high Km - captures the excess glucose for storage after a meal
serves as the glucose sensor for insulin release in B-islet cells
insulin-dependent
Km for GLUT 2
15 mM
this means that the liver will pick up excess glucose after a meal and store it
GLUT 4
glucose transporter located in adipose tissue and muscle
responds to the glucose concentration in peripheral blood
insulin-dependent transport (increased insulin increases the number of transporters
how does insulin affect GLUT4?
stimulates the movement of additional GLUT 4 transporters to the membrane
Km for GLUT 4
5 mM (close to normal blood glucose level)
GLUT 4 is saturated when blood glucose levels are just a bit higher than normal
how does the liver utilize excess glucose?
uses glycolysis - excess glucose is converted to fatty acids for storage
how do the beta-islet cells in the pancrease know when to release insulin?
GLUT2 begins to transport glucose into the cell
glucokinase is induced by insulin; phosphorylates glucose -> G6P
what is the first step in Glucose Metabolism?
transport across the membrane (facilitated diffusion/active transport) & phosphorylation by kinase enzymes inside the cell to prevent glucose from leaving via the transporter
glucose —> glucose 6-phosphate

Hexokinase
widely distributed enzyme in tissues
glucose –> glucose 6-phosphate
Low Km (reaches vmax at low [glucose])
inhibited by G6P

Glucokinase
only in liver cells and pancreatic B-islet cells
glucose —> glucose 6-phosphate
induced by insulin in the liver (acts as a glucose sensor)
High Km (acts proportionally to [glucose])

Phosphofructokinase-1
rate-limiting enzyme and main control point in glycolysis
fructose 6-phosphate –> fructose 1,6 biphosphate
inhibited by ATP, citrate, and glucagon (indirectly)
(glycolysis shouldn’t be on if we have enough energy)
activated by AMP and insulin (indirectly)
(glycolysis should be on if we need energy)
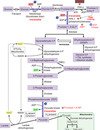
Phosphofructokinase-2
directly activated by insulin
converts a little fructose 6-phosphate —> fructose 2,6-bisphosphate (which activates PFK-1)
directly inhibited by glucagon, lowering F2,6-BP, inhibiting PFK-1
found mostly in the liver
can override the PFK-1 inhibition caused by ATP so that glycolysis can continue

Glyceraldehyde 3-phosphate dehydrogenase
catalyzes an oxidation and addition of Pi to substrate
glyceraldehyde 3-phosphate —>1,3-bisphosphoglycerate (high-energy intermediate)
reduction of NAD+ —> NADH

what is the difference between substrate-level and oxidative phosphorylation?
substrate level: ADP is directly phosphorylated to ATP using a high energy intermediate
oxidative: dependent on O2; ATP made from electron transport and chemiosmosis
3-Phosphoglycerate kinase
moves high energy Pi from 1,3-biphosphoglycerate (G3P dehydrogenase) to ADP –> ATP and 3-phosphoglycerate
3-phosphoglycerate –> 1,3 bisphosphoglycerate

Pyruvate Kinase
activated by fructose 1,6-bisphosphate from the PFK-1 reaction
an example of feed-forward activation
phosphoenolpyruvate (PEP) + ADP —> pyruvate + ATP

Lactate Dehydrogenase
key fermentation enzyme in mammalian cells when O2 or mitochondria are absent
oxidizes NADH to NAD+ (replenishing for G3P dehydrogenase)
reduces pyruvate to lactate
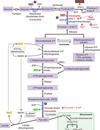
Dihydroxyacetone phosphate (DHAP)
glycolysis intermediate
used in hepatic and adipose tissue for triacylglycerol synthesis
formed from fructose 1,6-bisphosphate
can be isomerized to glycerol 3-phosphate <—> glycerol (backbone of triacyglycerols)
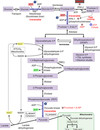
1,3-bisphosphate and phosphoenolpyruvate
high-energy intermediates used to generate ATP by substrate-level phosphorylation
only means of gaining ATP in anaerobic respiration

which enzymes in glycolysis are irreversible?
Hexokinase/Glucokinase, PFK-1, and Pyruvate kinase
How Glycolysis Pushes Forward the Process: Kinases
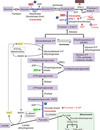
Bisphosphoglycerate Mutase
rearranges the phosphate in 1,3-BPG
1,3-bisphosphoglycerate —> 2,3 bisphosphoglycerate
Mutases
enzymes that move a functional group from one place in a molecule to another
2,3 bisphosphoglyerate (2,3-BPG)
binds allosterically to hemoglobin
decreases its affinity for oxygen
creates rightward shift in O2 disaccosication curve for hemoglobin
does not bind to fetal hemoglobin

how is fermentation in mammals different from fermentation in yeast?
in mammals, pyruvate is reduced to lactate
in yeast, pyruvate is converted to ethanol and CO2
Galactokinase
phosphorylates galactose and traps it in the cells
galactose —> galactose 1-phosphate

Galactose 1-phosphate Uridyltransferase
galactose 1-phosphate –> glucose 1-phosphate
this reaction also requires an epimerase
links galactose metabolism to glycolysis

Epimerase
enzymes that catalyze the conversion of one sugar epimer to another (differ at exactly one chiral center)
Fructokinase
phosphorylates fructose —-> fructose 1-phosphate, trapping it in the cell

Aldolase B
cleaves fructose 1-phosphate —> glyceraldehyde and DHAP
links fructose metabolism to glycolysis

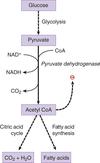
Pyruvate Dehydrogenase Complex
pyruvate + CoA + NAD+ —> acetyl-CoA + NADH + CO2
irreversible enzyme
present in the liver
activated by insulin (high insulin = well-fed state. liver should make energy, make fat, or store fat)
inhibited by its product acetyl-CoA
hight levels of acetyl-CoA implies that cell is satisified and need not enter citric acid cycle
(eventual buildup causes a shift from entering citric acid cycle or fatty acid oxidation to produce oxaloacetate for gluconeogenesis)
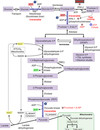
Three possible fates of pyruvate:
conversion to acetyl-CoA by pyruvate dehydrogenase
conversion to lactate by lactate dehydrogenase
conversion to oxaloacetate by pyruvate carboxylate
Glycogen
storage form of glucose
synthesized and degraded primarily by liver and skeletal muscle
stored in the cytoplasm as granules
granules have central protein core with polyglucose chains radiating outward to form a sphere
can be branched or linear chains

how is the structure of glycogen granules different when they are composed of linear chains and branched?
when the chains are linear, the highest density of glucose is near the core
when the chains are branched, the highest density of glucose is near the periphery, allowing more rapid release of glucose

Glycogenesis
synthesis of glycogen granules using glycogen synthase and branching enzyme
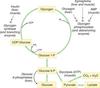
what is the mechanism of glycogenesis?
begins with a core protein, glycogenin, to which glucose is added
glucose —> glucose 6-phosphate —> glucose 1-phosphate
glucose 1-phosphate is activated by coupling to a molecule of uridine diphosphate UDP
glucose 1-phosphate + UTP —> UDP-glucose + pyrophosphate (PPi)
once activated, glucose can be added to glycogen chain

Glycogen synthase
rate-limiting enzyme of glycogen synthesis
UDP-glucose —> glycogen
forms alpha-1,4 glycosidic bond found in the linear glucose chains of the glycogen granule
activated by glucose 6-phosphate and insulin in liver and skeletal
inhibited by epinephrine and glucagon

Branching enzyme
responsible for introducing alpha-1,6 linked branches into the glycogen granule as it grows
- hydrolyzes one of the alpha-1,4 bonds to release a block of oligoglucose (relocated to another position)
- forms an alpha-1,6 bond to create a branch

Glycogenolysis
process of breaking down glycogen using glycogen phosphorylase and debranching enzyme

Glycogen phosphorylase
rate-limiting enzyme of glycogenolysis
glycogen —> glucose 1-phosphate
glucose 1-phosphate is converted to glucose 6-phosphate by mutase
breaks alpha-1,4 glycosidic bonds, releasing glucose 1-phosphate from the periphery of the granule
cannot break alpha-1,6 bonds so it can only degrade linear chains
activated by glucagon (liver), AMP (skeletal), and epinephrine
inhibited by ATP

Debranching enzymes
two-enzyme complex that deconstructs the branches in glycogen that have been exposed by glycogen phosphorylase
- breaks an alpha-1,4 bond adjacent to the branch point
- moves the small oligoglucose chain that is released to the exposed end of the other chain
- forms a new alpha-1,4 bond
- hydrolyzes the alpha-1,6 bond, releasing the single residue at the branch point as free glucose

Important substrates for gluconeogenesis:
glycerol 3-phosphate (from triacylglycerols)
lactate (from anaerobic glycolysis)
glucogenic amino acids (from muscle proteins)
(also dietary fructose and glucose)

which enzymes in gluconeogenesis are irreversible?
pyruvate carboxylase
phosphoenolpyruvate carboxykinase
fructose-1,6-bisphosphatase
glucose-6-phosphatase
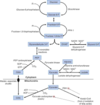
Pyruvate Carboxylase
mitochondrial gluconeogenic enzyme
pyruvate —> oxaloacetate (citric acid cycle intermediate stuck in mitochondria, reduced to malate)
once in the cytoplasm, oxaloacetate is made again from malate
works with phosphoenolpyruvate carboxykinase to replace pyruvate kinase
activated by acetyl-CoA (fom beta-oxidation)
high levels of acetyl-CoA suggest cell satisified, so pyruvate shunted to pyruvate carboxylase to generate more glucose for the rest of the body
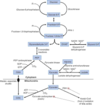
Phosphoenolpyruvate carboxykinase
gluconeogenic enzyme
converts OAA –> phosphoenolpyruvate (rxn requires GTP)
works with pyruvate carboxylase to replace pyruvate kinase
PEP later forms fructose 1,6-bisphosphate
induced by glucagon and cortisol to raise blood glucose levels
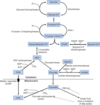
Frutose 1,6-bisphosphatase
key control point of gluconeogenesis; rate-limiting step of the process
fructose 1,6 bisphosphate –> fructose 6-phosphate (opposite of PFK-1)
activated by ATP
(high levels imply cell is energetically satisified and doesn’t need to break down glucose)
inhibited by AMP and fructose 2,6 bisphosphate
high levels of AMP mean cell needs to break down glucose
F2,6-BP is produced by PFK-2 to activate PFK-1 (levels increased with insulin, decreased with glycogen)

Fructose 2,6 bisphosphate
marker for satisfactory energy levels in liver cells
low levels signals to the liver that it should shift its function from burning energy to storing energy
controls both gluoconeogenesis and glycolysis
glucagon lowers levels to stimulate gluconeogensis
insulin increases levels to stimulate glycolysis

Glucose 6-phosphatase
gluconeogenic enzyme found only in the ER of liver cells
glucose 6-phosphate —> glucose diffuses into cytoplasm
is used to circumvent glucokinase and hexokinase
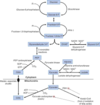
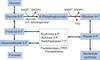
Pentose phosphate pathway / hexose monophosphate (HMP) shunt
occurs in the cytoplasm of all cells
produces NADPH and serves as a source of ribose 5-phosphate for nucleotide synthesis
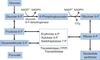
Glucose-6-phosphate dehydrogenase
irreversible rate-limiting enzyme involved in the first step of the pentose phosphate pathway
glucose 6-phoshate to make NADPH
induced by insulin (high [glucose] entering the cell) and NADP+
inhibited by NADPH
Functions of NADPH
biosynthesis of fatty acids and cholesterol
assisting in cellular bleach production in certain white blood cells
maintenance of a supply or reduced glutathione to protect against reactive oxygen species


