Lab test #1 Flashcards
(51 cards)
ms parts

magnification levels
4x = scanning 10x = low power 40x = high power or high dry 100x = oil immersion
ms part: ocular
the lens at the top of the microscope that you look through. They eyepiece is usually 10x or 15x power.
ms part: Tube
connects the eyepiece to the objective lenses.
ms part: Arm
supports the tube and connects it to the base of the microscope.
ms part: Base
the bottom of the microscope, used for support.
ms part: light
a steady light source (110v) used in place of a mirror. If your microscope has a mirror, it is used to reflect light from an external light source up through the bottom of the stage.
ms part: stage
the flat platform where you place your slides. Stage clips hold the slides in place. If your microscope has a mechanical stage, you will be able to move the slide around by turning two knobs. One moves it left and right, the other moves it forward and back.
ms part: revolving nosepiece
this is the part of the microscope that holds two or more objective lenses and can be rotated to easily change power (magnification).
ms part: objective lenses
usually you will find 3 or 4 objective lenses on a microscope. They almost always consist of 4x, 10x, 40x and 100x powers. When coupled with a 10x (most common) eyepiece lens, we get total magnification of 40x (4x times 10x), 100x, 400x, and 1000x.
ms part: condenser lens
the purpose of the condenser lens is to focus the light onto the specimen. Condenser lenses are most useful at the highest powers (400x and above). Microscopes with a stage condenser lens render a sharper image than those with no lens (at 400x). If your microscope has a maximum power of 400x, you will get the maximum benefit by using a condenser lenses rated at 0.65 NA or greater. 0.65 NA condenser lenses may be mounted in the stage and work quite well. A big advantage to a stage mounted lens is that there is one less focusing item to deal with. If you go to 1000x then you should have a focusable condenser lens with an N.A. of 1.25 or greater. Most 1000x microscopes use 1.25 Abbe condenser lens systems. The Abbe condenser lens can be moved up and down. It is set very close to the slide at 1000x and moved further away at the lower powers.
ms part: diaphragm or iris
Many microscopes have a rotating disk under the stage. This diaphragm has different sized holes and is used to vary the intensity and size of the cone of light that is projected upward into the slide. There is no set rule regarding which setting to use for a particular power. Rather, the setting is a function of the transparency of the specimen, the degree of contrast you desire and the particular objective lens in use.
Parfocal microscope
objectives stay in focus when magnification is changed; i.e., if the microscope is switched from a higher power objective (e.g., 40×) to a lower power objective (e.g., 10×), the object stays in focus.
1.8mm is equal to how many microns?
.4 mm is equal to how many microns?
1800 microns
400 microns
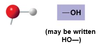
-OH
Hydroxyl
CH3
Methyl

NH2
Amine

-COOH
Carboxyl

-CHO
carbonyl (aldehyde)

=CO
Carbonyl (ketone)

-SH
sulfhydryl

H2
molecular hydrogen

CH3OH
Methyl Alcohol

O2
molecular oxygen













