The hormones that control metabolism week 4 Flashcards
Which of the following hormones are catabolic? Which are anabolic?
glucagon
thyroid hormone
insulin
catecholamines
glucocorticoids
testosterone
Catabolic hormones: glucocorticoids, catecholamines, glucagon
Anabolic hormones: insulin, testosterone, thyroid hormones
List the 3 parts of the pituitary that the hypothalamus innervates.
posterior pituitary
median eminence
anterior pituitary
T or F: Endocrine glands fxn as more or less independent entities but are organized in a system that integrates their activity and interfaces them to the CNS.
True.
The ____ and the ____, acting together, control the fxn of a number of endocrine glands that contribute to the regulation of metabolism.
hypothalamus and pituitary
Explain the functions of each of the parts of the pituitary.
- Posterior pituitary (neuro-hypophyse)
- axonal transport of neurohormones
- storage in secretory vesicles
- release into bloodstream
- peripheral target cells - Median eminence (neurovascular region)
- hormones are release into bloodstream
- neurohormones reach anterior pituitary through local circulation - Anterior pituitary (adeno-hypophyse)
- endocrine cells synthesize, store, and
secrete peptides and hormones
- release into bloodstream
- paracrine & endocrine function
What hormones are contained within each of the 3 parts of the pituitary?
- in the Posterior pituitary (neuro-hypophyse)
- ADH: Antidiuretic hormone (vasopressin)
- OCT: Oxytocin
- in the Median eminence (neurovascular region)
- TRH: Thyrotropin releasing hormone
- CRH: Corticotropin releasing hormone
- GHRH: Growth hormone releasing &,inhibiting hormone (somatostatin).
- GnRH: Gonadotropin releasing h.
- PRF: Prolactin releasing & inhibiting factor
- From the anterior pituitary hypothalamic hormones release :
- ACTH: Adrenocorticotropic hormone
- TSH: Thyroid-stimulating hormone
- LH: Luteinizing hormone
- FSH: Follicle-stimulating hormone
- PRL: Prolactin
- GH: Growth hormone (somatotropin)
- MSH: Melanocyte-stimulating h.

Explain the ultrashort, short, and long feedback loops of the hypothalamic pituitary-target
organ axis.

What are the physiological fxns of the adrenal glands?

What embryonic layer is the adrenal cortex derived from? What are the 3 parts of the adrenal cortex? What is synthesized in each of the layers of the adrenal cortex?
What is the embryonic origin of the adrenal medulla? What is synthesized in the adrenal medulla?
• Cortex (mesoderm)
– corticosteroids
a. Zona glomerulosa: Mineralocorticoid (Aldosterone)
b. Zona fasiculata: Glucocorticoid (Cortisol)
c. Zona reticularis: Sex steroid precursor (andosterones)
• Medulla (neural crest)
– catecholamines
a. Epinephrine
b. Norepinephrine

Where do hormones synthesized in the cortex have to go to reach the body?
Cortex hormones diffuse through medulla to reach the central vein.

How is cholesterol stored in the adrenal cortex?
What is the cellular localization of steroid hormone syntheis?
What enzyme catalyzes steroid hormone synthesis? What is required for its rxn?
When are steroid hormones produced?
Cholesterol is taken up into cells. It is esterified and stored into vesicles. Steroid hormones are synthesized in the ER and mitochondria by CYP11A1 (desmoslase) using NADPH and O2.
Corticosteroids are not stored. Synthesis requires stimulation of the gland. When stimulated, cholesterol is transported to the proper organelles for hormone synthesis.
The formation of what molecule is rate limiting in steroid hormone synthesis in the mitochondria?
What modification occurs to progesterone? In what organelle is this performed?
What is the immediate precursor to cortisol? To what cellular oganelle is it transported to form cortisol? What modification is made?
- Cortisol synthesis: Zona Fasciculata
- Pregnenolone formation is rate limiting in mitochondria
- Progesterone hydroxylation in the ER
- 11-deoxycortisol transferred to mitochondria. critical C11 hydroxylation to form cortisol

What is the precursor to aldosterone? What must be done to its precursor to form aldosterone? What is the cellular localization of this rxn?
Corticosterone is hydroxylated to form the immediate precursor to aldosterone in the mitochondria.

How is cortisol transported in the blood? What form (bound or unbound) is active?
How does cortisol enter cells? Once there, what does it do?
What are the effects of cortisol on metabolism? (what is its fxn?)
During what times is cortisol important?
What hormone does cortisol oppose?
- The major role of Cortisol is the regulation of metabolism and the organism’s response to stress. The latter makes it essential for life. In the plasma, transcortin transports cortisol but only free cortisol is active. Cortisol can be derived from Cortisone and both are entirely metabolized in the liver.
- Cortisol enters the cell via facilitated diffusion. Its binding to a cytoplasmic cortisol receptor promotes receptor phosphorylation and its nuclear translocation. Histone acetylation in the nucleus leads to changes in transcription.
- Cortisol on metabolism: Cortisol stimulates the conversion of protein to glucose (proteolysis) and storage as glycogen (glycogenesis). The mobilized protein derives mostly from muscle stores leading to decrease in muscle, connective tissue, skin mass. Cortisol promotes fat lipolysis, stimulates appetite and lipogenesis.
- increases blood glucose for vital organs
- increases glomerular filtration, increased urine production (blocks ADH), increases blood pressure (increases sensitivity of cells to B-adrenergic signaling), suppresses immune response
- Glucocorticoid function is important during fasting and hypoglycemia.
- Cortisol opposes insulin action by inhibiting insulin dependent glucose uptake and suppression of glucose release from the liver.

Explain how cortisol is released from the adrenal cortex.
How is cortisol release regulated? (what feedback loops are involved?)
Corticotropin Releasing Hormone (CRH) stimulates
Adrenocorticotropic hormone (ACTH)
- produced by corticotroph cells
-co-secreted with melanocyte stimulating hormone
- stimulates Cortisol release (adrenal androgens, aldosterone)
Short loop : ACTH
- inhibits CRH release
Long loop : Cortisol
- blocks ACTH and CRH release (min)
- attenuates ACTH synthesis (hrs). this effect is observed with prolonged cortisol exposure.
Antidiuretic hormone (ADH)
- released from posterior pituitary
- potent stimulator of ACTH
- cortisol negatively regulates ADH release

What effect do synthetic glucocorticoids have on CRH and ACTH release? On the adrenal cortex?
Synthetic Glucocorticoids (dexamethasone, prednisone, fludrocortisone, hydrocortisone)
- all have negative feedback on CRH and ACTH secretion
- ACTH dependent adrenal cortex atrophies (dc cortisol production results)
What effects does ACTH have on the adrenal gland?
ACTH stimulates:
- cAMP production
- stimulates PKA, PKC
Synthesis:
- steroidogenesis – hydrolysis of Cholesterol ester
- transport of cholesterol to mitos
Transcription:
- activation of gene transcription
- increase enzymes for Cortisol synthesis
Growth:
- increase in cell size & number
- hyperplasia
Trophic effects of ACTH on the adrenal cortex
a. ACTH stimulates cell growth and proliferation.
b. A deficiency of ACTH greatly reduces hormone production by the adrenal cortex.
c. An excess of ACTH leads to hyperplasia of the adrenal cortex.

What other type of receptor can cortisol bind to when present in high concentrations?
mineralocorticoid receptors

At what time are ACTH and cortisol levels the highest? What is the timing of the release of CRH, ACTH, and cortisol?

What effect does stress have on cortisol release?
- stress overrides the diurnal pattern
- stress can attenuate the negative feedback
- while exercise is a stressor, training reduces cortisol release in response to exercise
- low exercise responders are also low stress responders

What is Cushing’s disease? What is it caused by? What are the sx of Cushing’s disease?
• enhanced Cortisol levels
– ACTH secretion from pituitary adenoma (70%) C. Disease
– ectopic ACTH from non-pituitary tissue (15%)
– adrenal adenoma, hyperplasia (15%) (normal [ACTH])
• Hypertension (β-adrenergic)-cortisol increases sensitization to B-adrenergic stimulation
• weight gain (upper body) fat redistribution-due to water retention
• Hyperglycemia, polyuria-mobilization of proteins and lipids
• muscle weakness, skin thinning
• immune suppression!

What is Addison’s disease? What is it caused by? What are the sx?
Addison’s Disease: adrenal atrophy leading to aldosterone AND cortisol deficiency
• hypoglycemia, weight loss
• hypotension (hyponatremic)
• Hyperkalemia
• skin hyperpigmentation (ACTH is co-secreted with MSH)

What cells of the adrenal medulla synthesize norepinephrine and epinephrine? Which is secreted in higher proportions?
What is the precursor to E and NE synthesis?
How are E and NE stimulated for release?
What is packed in granules with E and NE?
What is the half life for E and NE? Where are they metabolized? How are they excreted?
The chromaffin cells of the adrenal medulla synthesize and secrete the catecholamine hormones; they are innervated by pre-ganglionic neurons (ACh is released from the preganglionic neuron onto nicotinic receptors present on chromaffin cells) that are a part of the sympathetic nervous system.
The adrenal medulla produces catecholamine hormones, norepinephrine and epinephrine. The precursor for their synthesis is tyrosine.
Chromaffin granules contain catecholamines, ATP, chromogranin and other neuropeptides.
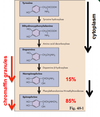
The adrenal medulla secretes 85% E and 15% NE. Most of the circulating NE is from sympathetic nerve terminals.
- Both NE and E:
- short half life of 2 minutes
- metabolized in liver & kideney
- secreted in the urine
What are the effects of E and NE release? (fight or flight response)
The sympathetic nervous system is stimulated by hypoxia, hypovolemia, and hypoglycemia













