Intro to carb metabolism glycolysis week 2 Flashcards
(31 cards)
What are the 4 main monosaccharides we consume? What kinds of carbs are they? (aldoses, ketoses)
How are carbons in carbohydrates numbered? What is the reducing end of a sugar?
What are the 2 types of glycosidic bonds?
What type of enzymes hydrolyze glycosidic bonds?
- glucose, mannose, and galactose are all aldoses (contain aldehyde groups). fructose is a ketose (contains keto group)
- Carbons are numbered starting from the reactive group-hydroxyl coming from aldehyde or keto part of molecule. This is called the reducing end of the sugar.
- There are 1,4 and 1,6 glycosidic bonds. This means there is a bond with the first carbon of one sugar with either the 4th or 6th carbon of another sugar.
- Glycosidases hydrolyze glycosidic bonds.
see slide 1 of notes
What are the 4 major pathways of carb metabolism? (just list)
glycolysis
gluconeogenesis
glycogenesis
glycogenolysis
What is glucose converted to in glycolysis? What is the net ATP production?
What is gluconeogenesis? When does it occur?
What are glycogenesis and glycogenolysis? When do they occur?
- In glycolysis, glucose is converted to pyruvate, some of the energy is extracted and the stage is set for complete oxidation to CO2 and H2O (or for reduction to lactate). Along the glycolytic pathway there is a net production of 2 ATPs. (Much more ATP energy is produced when glycolysis is followed by the Krebs cycle and terminal oxidation.)
- De novo synthesis of glucose is gluconeogenesis. Gluconeogenesis occurs when glucose level in the blood is falling (during fasting) and glucose is needed for certain organs (e.g. brain).
- When glucose coming from diet is more than that is needed for glycolysis, it is stored in the form of glycogen. Glycogen synthesis is called glycogenesis.
- Glycogen is degraded when glucose is needed for the cells. This is glycogenolysis.
Glycogen
Glycogenolysis ↓↑ Glycogenesis
Glucose
Glycolysis ↓↑ Gluconeogenesis
Lactate
What are the minor pathways of carb metabolism? What are their purposes?
- Glucose can be utilized in the hexose monophosphate shunt (pentose monophosphate pathway) to produce ribose phosphate for nucleotide synthesis, and NADPH that supplies reducing power for biosynthetic reactions (e.g. fatty acid synthesis). The intermediates in this pathway can also be shuttled to other pathways.
- Sugar derivatives and complex sugars can also be synthesized depending on the need of the cells. These derivatives can be used for the formation of glycoproteins, proteoglycans and glycolipids.

Glucose can be obtained from the diet and from storage. What is the stored from of glucose?
Dietary sources of carbs include monosaccharides, disaccharides, and polysaccharides. What is the composition of lactose, sucrose, and trehalose?
What are the major forms of polysaccharides humans can digest? What types of glycosidic bonds do they contain?
What carbs are indigestible? Why are they indigestible?
- Stored form of glucose is glycogen. Stored glycogen in our tissues is converted to glucose or glucose 6 phosphate by enzymes of glycogenolysis in the cells. Stored glucose is used for energy generation by glycolysis.
- monosaccharides: Fruits and honey contain free glucose and free fructose. These can be readily absorbed by enterocytes. Disaccharides in our diets include milk sugar (lactose) and table sugar (sucrose).
Lactose is composed of glucose and galactose.
Sucrose is composed of glucose and fructose.
Trehalose, found in mushrooms, is a di-glucose. - Major source of glucose is starch, a storage form of glucose in plants, which contains alpha-1,4-glycosidic linkages (forming amylose) along with alpha 1,6-glycosidic linkages (forming amylopectin). Glycogen is the storage form in animal tissues and contains the same type of linkages and branches.
- Indigestible carbs: Unusual linkage, cellulose, fiber. Do not have glycosidases that can hydrolyze these glycosidic linkages.

What enzyme digests the amylose chains (linear) of starch and glycogen? Whre is this enzyme located in the body? What are its products?
What enzyme hydrolyzes amylopectin (branched part of starch and glycogen)?
Where does further digestion of oligosaccharides occur? By what enzymes? (be specific)
After digestion, where do sugars go?
- Starch (in plants) and glycogen (in animals) are glucose polymers present in food. The amylose chains (linear) of these molecules are degraded by the enzyme alpha-amylase (an exoglycosidase), which is present in saliva and more abundant in pancreatic juice. The products are glucose, maltose (a disaccharide composed of 2 glucoses with a 1,4 glycosidic bond) and maltotriose (a trisaccharide). Amylopectin (branched part) is hydrolyzed by isomaltase (hydrolyzes glucose bound by 1,6 glycosidic bonds).
- Further digestion of the oligosaccharides occurs on the surface of the intestinal epithelial cells by alpha-glucosidases (maltase). Lactose is digested by lactase and sucrose by sucrase.
- Sugars go the portal circulation after digestion where they are then released into the body by the liver.

What is the best diet for individuals with liver disease?
What is a good diet for individuals with pancreatic issues?
- The best diet for liver disease is one composed of carbs because the liver is not involved in its digestion (does not have to produce bile to digest carbs)
- It is best to consume foods with mono and disaccharides-do not require lots of pancreatic amylase
What happens to indigestible carbs?
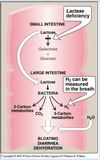
What 3 things is a hydrogen breath test used to diagnose?
What is disaccharide intolerance? What are causes of it?
What is lactose intolerance?
What sugar does isomaltase-sucrase deficiency result in the intolerance of?
- Any undigested disaccharide goes to the large intestine and causes osmotic diarrhea. Oligosaccharides not hydrolyzed by amylase or the intestinal surface enzymes are not degraded and reach the lower ileum where bacteria, with a greater range of saccharidases, metabolize the sugars anaerobically to produce short –chain fatty acids, lactate, H2, methane and CO2 (gas!)
- Hydrogen breath test used for
• Bacterial overgrowth in the small intestine
• Digestive problems, lactose intolerance
• Rapid passage of food through the small intestine - Disaccharide intolerance, the loss of brush border enzymes, can be acquired by the variety of intestinal diseases, malnutrition or drugs that injure the mucosa of the small intestine. Temporary disorder can result from severe diarrhea.
- In case of lactose intolerance, when lactase is deficient, lactose will pass to the colon and will be digested by bacteria causing GI tract distress, such as cramping and bloating due to carbon dioxide and methane production. More than half of the world’s adult population is lactose intolerant. It is particularly widespread among Asian and African descent that are up to 90% lactase-deficient.
- Isomaltase-sucrase deficiency results in the intolerance of sucrose. About 10% of Greenland Eskimos and 2% of North Americans are heterozygous for the deficiency.
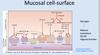
How do enterocytes obtain energy?
How are monosaccharides absorbed by enterocytes? (what form of transport is used?)
- These intestinal cells do not depend on glucose and obtain energy from glutamine metabolism.
- Monosaccharides are absorbed by the enterocytes by facilitated transport.
What sugars does SGLT-1 transport? On what surface is it located? How does this transporter work?
What sugars do GLUT-5 and GLUT-2 transport? Where are these transporters located?
How are pentoses (such as xylose) and L-sugars transported?
How is mannose absorbed?
What happens after glucose, galactose, fructose, and mannose enter portal circulation?
- Na-monosaccharide cotransporter (SGLT-1)- specific for glucose and galactose and carries Na-ion with the monosaccharides (secondary active transport, works against sugar concentration gradient, driven by sodium ion gradient and coupled with ATP hydrolysis-Na+/K+ pump). Note that if the Na+/K+ pump is malfunctioning, SGLT-1 will not work.
- Na-independent monosaccharide transporters (GLUT): GLUT-5 is specific for fructose. GLUT-2 accepts all three monosaccharides and located on the contraluminal plasma membrane. Energy from the environment (heat for example) drive facilitated transport.
- Pentoses and L-sugars enter by passive transport.
- We eat very little mannose, which is taken up by a Na-dependent transporter, which is not the same as the Na-monosaccharide co-transporter above.
- Monosaccharides then enter the portal blood and are delivered to the liver. Glucose is distributed to the general circulation, while galactose, fructose and mannose are processed mainly by the liver.


What are 7 fxns of glycolysis?
Functions of glycolysis:
- Energy-yielding pathway; yields 2 ATPs from 1 glucose (anaerobic process; cornea, lens, retina, red blood cells that have no mitochondria, will rely on these 2 ATPs)
- Sets the stage for aerobic oxidation of carbohydrates (the end product, pyruvate, can be transformed to AcCoA, which will enter the Krebs cycle)
- Supplies intermediates for carbohydrate storage (in the form of glycogen)
- Supplies intermediates for the pentose phosphate pathway (synthesis of NADPH and 4-C, 5-C, 7-C, sugars)
- Supplies intermediates for 2,3-BPG synthesis (regulator of oxygenation in RBC)
- Supplies intermediates for special carbohydrate synthesis (glucoronate, glucosamine-6- P, etc)
- Can take up glycerol from triacylglycerols.

What is anaerobic fermentation? What process in the body is a type of anaerobic fermentation?
The Embden-Meyerhof or glycolytic pathway is an ancient process possessed by all cells of the human body in which anaerobic degradation of glucose to lactate occurs. This is also called anaerobic fermentation, a process by which chemical energy is extracted from high-energy fuels in the absence of oxygen.
Glycolysis is an emergency energy-yielding pathway capable of yielding 2 ATP molecules from 1 glucose molecule when oxygen is not present. Thus, when oxygen is shut off, ATP levels can still accumulate over a short period of time. When is this especially important?
Glycolysis is especially important at birth when circulation of the blood decreases to most parts of the neonate during delivery except the brain. This conserves oxygen for use by the brain.
Explain the listed tissues need for glycolysis and whether or not they have mitochondria:
brain
RBCs
cornea, lens, retina
kidney medulla
testis
WBCs
white muscle fibers
red muscle fibers
heart muscle
- The brain has an absolute need for glucose and processes most of it via glycolysis. The end product is pyruvate. The pyruvate produced is then oxidized to CO2 and H2O in the mitochondria (in the Kreb cycle).
- In other cells, lactate is the end product.
- Red blood cells lack mitochondria and are unable to metabolize pyruvate.
- The cornea, lens and regions of the retina have a limited blood supply and also lack mitochondria (mitochondria would absorb and scatter light) and depend on glycolysis.
- Kidney medulla, testis, leukocytes and white muscle fibers are almost totally dependent on glycolysis because the cells have few mitochondria.
- Skeletal muscle cells also run glycolysis. The end product is pyruvate under aerobic conditions and lactate under anaerobic conditions. Heart muscle works under aerobic conditions, but its major fuel is fatty acid.

What 3 stages can glycolysis be broken into?

How many ATPs are hydrolyzed during the priming stage (energy investment stage)?
What are the ATPs used for?
Priming stage (energy investment phase) involves input of 2 ATPs to convert glucose to fructose 1,6-bisphosphate. This investment gives a return later.
What 2 steps/enzymes are regulated in the priming phase of glycolysis? (just list)
hexokinase (glucokinase in liver-isozyme that catalyzes same rxn but has slightly diff amino acid sequence)
PFK-1
What is the first regulation step in glycolysis? What is the product of this reaction? What is significant about the product of this rxn?
hexokinase (in addition in liver glucokinase): 1st regulation step. Catalyzes rxn of glucose to glucose 6 phosphate.
Glucose is trapped as glucose 6-phosphate within the cytosol through phosphorylation, which blocks penetration through the membrane. The reaction is favorable and irreversible under cellular conditions.
Glucose-6-phosphate is a junction point in metabolism. The fate of glucose is not yet determined (see attached).

What is the 2nd regulation step in glycolysis? What rxn occurs in this step?
This step is the most heavily regulated in glycolysis. Why?
6-phophofructo-1-kinase (PFK1) 2nd regulation step. Catalyzes rxn of fructose 6-phosphate to fructose 1,6-bisphosphate
committed step and rate-limiting step of glycolysis
This enzyme is subjected to extensive regulation and is irreversible under intracellular conditions.

What 2 steps occur during the splitting stage? What enzymes are involved?
What isozyme of an enzyme (involved in these 2 steps) is important for fructose metabolism?
The splitting stage splits a 6-carbon sugar into two 3 carbon sugars and yields 2 molecules of glyceraldehyde 3-phosphate.
a. aldolase: F 1,6-bisphosphate aldolase catalyzes cleavage of F 1,6-bisphosphate into one dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-P (GAP). This step is reversible.
b. triose-P isomerase catalyzes reversible conversion of DHAP to GAP. We get two GAPs from one glucose molecule. The reaction is freely reversible and can be used for glycolysis and gluconeogenesis.
Aldolase-B splits fructose 1-P in fructose metabolism

What are 4 important steps that occur in the oxidoreduction-phosphorylation stage?
State enzymes used, products, where ATP produced, etc.
What rxn is the 3rd regulated step of glycolysis?
glyceraldehyde 3-P dehydrogenase (GAPDH)
-high-energy P formation
-2 NADH produced/glucose
GAP goes to 1,3 bisphosphoglycerate. GAPDH oxidizes the aldehyde group to a carboxylic acid with reduction of NAD+ to NADH. The 1,3 BPG is highly energetic and can yield ATP in the next reaction. This reaction is freely reversible in the cells and is used in both glycolysis and gluconeogenesis. This is an important step if running glycolysis under anaerobic conditions: 2 NADH=3ATP in electron transport chain
phosphoglycerate kinase
-1st substrate-level phosphorylation
-2 ATPs produced/glucose
1,3-bisphosphoglycerate to 2,3, bisphosphogylerate. ATP is made by using the energy of 1,3-bisphosphoglycerate. This is the first site of ATP production. The ATP used earlier (phosphofructokinase) is recovered. This is an example of substrate level phosphorylation because it occurs with substrate without involvement of oxidative phosphorylation. The reaction is freely reversible and can be used for glycolysis and gluconeogenesis.
a phosphatase removes phosphate from 2,3, bisphosphoglycerate to from 3-phosphoglycerate. 3-phosphoglycerate is converted to 2 phosphoglycerate.
enolase: converts 2-phosphoglycerate to phosphoenolpyruvate (PEP). high-energy phosphate bond is generated The reaction is freely reversible and can be used for glycolysis and gluconeogenesis.
pyruvate kinase
-2nd substrate-level phosphorylation
-3rd regulation step
-2 ATP produced/glucose
Pyruvate kinase catalyzes another substrate level phosphorylation and converts PEP into pyruvate. The reaction is not reversible under physiologic conditions and so PEP cannot be made for synthesis of glucose.
attached is slide 21 of notes

What shunt is present in RBCs that uses 15-25% of glucose? What enzyme and product is involved? What does this mean for ATP generation?
What is the purpose of this shunt?
In red blood cells, 2,3-BPG is generated by the 2,3 bisphosphoglycerate mutase/phosphatase bifunctional enzyme, which uses 1,3-bisphospho-D-glycerate as substrate. This enzyme can also convert 2,3-BPG to 3 PG. RBCs contain very high level of 2,3-BPG because it is a negative allosteric effector of association of oxygen with Hb. From 15-25% of the glucose taken up by the RBCs goes to the BPG shunt, which generates no net ATP since the phosphoglycerate kinase step is bypassed.

What is the endproduct of glycolysis (pyruvate) converted to under anaerobic conditions? What enzyme catalyzes this rxn? What is produced in this rxn?
Explain the reversibility of this rxn.
Lactate dehydrogenase–> NAD+regenerated
Lactate dehydrogenase reduces pyruvate to lactate and NADH (generated at GAPDH) is oxidized to NAD+. This reaction is freely reversible and the only reaction to make lactate or utilize lactate. It can be used by both glycolysis and gluconeogenesis. Note that the reversibility of this reaction allows lactate to reform pyruvate, which can be shunted into the Krebs cycle. Thus, when lactic acid accumulates in muscle due to lack of oxygen, with time, as oxygen is redistributed into the tissue, it will reverse LDH and turn on PDH and the Krebs cycle and pull lactate into energy generation.








