Nucleotide metabolism week 4 Flashcards
nucleoside
nucleotide
nucleic acid
Nitrogen-containing bases
Nucleoside = base + sugar (ribose or deoxyribose)
Nucleotide = nucleoside phosphates (mono-, di-, tri-phosphate)
Nucleic acid = polymer of nucleotides
Name the nucleosides and nucleotide of the following bases:
Adenine
Guanine
Hypoxanthine
Xanthine
Cytosine
Uracil
Thymine
Orotic acid

What are the functions of nucleotides?

What are the 3 sources of nucletoides? What is the source of most of our nucleotides?
1) Diet (5%)
2) de novo synthesis
3) salvage from existing bases
Majority come from salvage. synthesis is expensive so recycle nucleotides a lot. Also, not all cells can synthesize nucleotides.
What pancreatic enzymes are responsible for nucleotide degradation? Where do nucleotide degradation products go after action of pancreatic enzymes?
Pancreatic enzymes:
Nucleases –> oligonucleotides
Phosphodiesterases –> mononucleotides
Nucleotidases –> nucleosides
Nucleosidases –> bases
Sugar units and bases are absorbed. Sugars and small amounts of bases (5% of total) enter circulation. 95% of bases are degraded in the intestinal mucosal cells.

What are the pathways for nucleotide metabolism?
- Ribonucleotide metabolism
a. Purine de novo synthesis
b. Purine salvage pathway
c. Purine degradation
d. Pyrimidine de novo synthesis
e. Pyrimidine salvage pathway
f. Pyrimidine degradation - Deoxynucleotide synthesis
How many ATP are needed for de novo synthesis of purines and pyrimidines?
De novo synthesis of both purines and pyrimidines is complex and expensive:
Purine synthesis requires 11 steps and 6 molecules of ATP
Pyrimidine synthesis requires 9 steps and 6 molecules of ATP
Consequently, both purines and pyrimidines have salvage pathways to “rescue” bases from degradation and return them to the nucleotide pool
Within non-dividing cells, what is the dominant nucleotide? When are deoxynucleotides synthesized and what molecule is required for synthesis?
Within non-dividing cells, most of the nucleotides present are ribonucleotides. When cells enter the S phase of mitosis, they synthesize deoxyribonucleotdies for DNA replication, which need to be synthesized from ribonucleotides.
What molecules are required for purine synthesis? What molecule is purine synthesis initiated on?
What is the cellular localization of the enzymes required for purine synthesis?
What is the first purine formed? What 2 purines are synthesized from this first purine?
How many ATP are required? In what steps is ATP utilized?
- Purine ring forms from carbon dioxide and amino acids on PRPP (5-phosphoribosyl-1-pyrophosphate), an activated ribose base. Glycine, Glutamine, and Aspartate are required for this process. Aspartate and Glutamine donate nitrogens. (glutamine binds to PRPP first). Glycine donates its whole structure (minus the carboxyl group)
- R-5-P is produced from HMPS
- THF serves as C1 carrier (provides 2 carbons)
- A trifunctional, two bifunctional and three monofunctional enzymes are involved
- All enzymes are in the cytosol
- First purine is IMP
- AMP and GMP synthesized from IMP (serves as a branch point)
- 5 ATP equivalents are used for IMP, +1 ATP for GMP, +1 GTP for AMP (6 total ATP). Remember that this process is 11 steps.
- Note that carbs and aa must be available for this process (glycolysis). Energy producing pathways must also be occuring to meet ATP requirements.
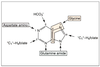
What enzyme is required to form PRPP from ribose-5-P? What does this enzyme require for this reaction?
Remember that PRPP is an activated ribose base.
ATP and Mg2+ are required for this reaction.

What is the rate limiting step in purine synthesis? What substrate is required and what is produced in this step?
How is this enzyme regulated?
After the formation of this product (after action of the rate limiting enzyme), what 2 intermediates are produced? What 2 enzymes are required? How are they regulated?
The rate limiting step is the formation of IMP from PRPP (which requires what was previously discussed and is noted in attached pic). This reaction is catalyzed by Glutamine phosphoribosyl amidotransferase.
Glutamine phosphoribosyl amidotransferase is stimulated by PRPP and is inhibited by IMP, GMP, and AMP.
GMP is produced from IMP by IMP dehyrogenase. This enzyme is inhbited by GMP.
AMP is produced from IMP by adenylosuccinate synthetase. This enzyme is inhibited by AMP.
ATP and GTP are then produced from AMP and GTP, respectively.

How does methotrexate inhibit purine synthesis? What enzyme does it inhibit? What is this drugs used for?
What enzyme does mycophenolic acid inhibit? What cell types does this drug act on? What is it used for?
Methotrexate – acts at steps where formyl-THF is used. It inhibits dihydrofolate reductase, regeneration of THF from DHF, and thus cell division. (chemotherapeutic drug).
Mycophenolic acid – Inhibits IMP dehydrogenase. It acts on rapidly proliferating T and B cells (anti graft rejection drug).

Explain the degradation of purines. What is the end product of purine degradation? What enzyme is required?
What does this enzyme produce? What is required for its activity?
Adenine nucleotides are metabolizied to hypoxanthine while guanine nucleotides are metabolized to xanthine. These purines are metabolized by xanthine oxidase to form uric acid.
O2 is needed for xanthine oxidase and NADH is produced.

What is gout? What are 3 causes of gout?
What are the differences btwn primary and secondary gout?
What 3 drugs may be used to treat gout? How do they work?
Gout: accumulation of uric acid in body fluids and joints (arthritic pain) or kidney. defect in purine degradation
Primary gout: idiopathic, can be caused by several genetic lesions (problem with purine metabolism)
Secondary gout: brought on by leukemia, polycysthemia, antimetabolite treatment of cancer, radiation therapy, renal insufficiency or consumption of excessive amount of alcohol or purine-rich food.
Uric acid is not very soluble in aqueous solutions; elevated levels of uric acid can result in deposition of sodium urate crystals primarily in joints.
-Allopurinol is an inhibitor of xanthine oxidase and can be used to block uric acid production. Hypoxanthine and xanthine are easier to excrete than uric acid. Only make uric acid because we cannot excrete adenosine and guanine.
-Colchicine – binds to polymorphonuclear leukocyte microtubules –> inhibits leukocyte locomotion, adhesiveness and phagocytosis. Inhibits inflammation but does not reduce urate level.
Plus anti-inflammatory drugs, such as aspirin, for pain relief.
-Uricosuric drugs, such as probenecid or sulfinpyrazone, to promote excretion of uric acid.

Hyperuricemia
Hyperuricuria
Hyperuricemia is excess levels of uric acid in the blood
- >1.5-6.0 mg/dl women
- >2.5-8.0 mg/dl in men
Hyperuricuria is excess levels of uric acid in urine
- 0.5-1gm/day
What substrates and enzymes are required for purine salvage? What is produced?
The “salvage” of bases requires the activity of phosphoribosyltransferases that utilize PRPP as the ribose phosphate donor. has an essential role, especially in extrahepatic tissues, where de novo nucleotide synthesis is normally low (depends on the circulating bases).

APRTase (Adenine phosphoribosyltransferase)
HGPRTase (Hypoxanthine–guanine phosphoribosyltransferase)
Hyoxanthine + PRPP–> IMP + PPi
Guanine + PRPP–> GMP + PPi
Adenine + PRPP–> AMP + PPi
What is Lesch-Nyan syndrome caused by? What is its inheritance pattern? When in life do sx of this disease onset?
What sx/diseases develop as a result of Lesch-Nyan syndrome? What is one way of treating this syndrom?
Lesch-Nyan syndrome is caused by defective HGPRT. Purine bases cannot be salvaged but are instead degraded. It is an X-linked recessive syndrome with an early age of onset.
Consequences:
• Purine synthesis is highly upregulated in liver.
• Megaloblastic anemia –> Elevated purine synthesis places great demand on tetrahydrofolate (THF) –> folate deficiency –> decreased ability to produce mature RBCs
• Accumulation of 5-aminoimidazole-4-carboxamide (orange crystals) intermediate in purine synthesis
• Purine catabolism is also upregulated
• Uric acid accumulates causing gout and kidney damage
• Death occurs due to kidney failure
• Neurological symptoms: extremely aggressive behavior, self-mutilation. Reasons are unknown. A dopamine-like NT level is decreased.
Treatment: allopurinol alleviates the symptoms of gout, but does not cure the disease.
What molecules are required for pyrimidine synthesis?
What is the cellular localization of the enzymes required for purine synthesis?
What is the first pyrimidine formed?
How many ATP are required? In what steps is ATP utilized?
What is a major difference btwn purine and pyrimidine synthesis?
- Pyrimidine ring forms first from carbamoyl phosphate and aspartate
- Carbamoyl phosphate is synthesized from Gln and CO2
- R-5-P is added in the form of PRPP
- A trifunctional enzyme – in cytosol
- A bifunctional enzyme – in cytosol
- A single enzyme – in mitochondrium
- First pyrimidine is UMP
- CTP and dTTP are synthesized after that
- 3 ATP equivalents are used for UMP, additional 2 ATPs are used for UTP and 1 ATP for CTP synthesis
Unlike the synthesis of purine bases which begins on PRPP, synthesis of pyrimidine bases begins with the synthesis of the base which is then attached to PRPP.

What are the 2 important enzymes in pyrimidine synthesis? What rxns do they catalyze?
What is the regulated enzyme in pyrimidine synthesis? What is it regulated by?
What is the cellular location of the regulated enzyme?
- Carbamoyl phosphate synthase II (CPS II): found in the cytosol of cells throughout the body. CPS II is the only source of carbamoyl phosphate in extrahepatic tissues. (CPS I is found in the mitochondria and fxns primarily in the liver in urea production)
CPS II is inhibited by UTP and is stimulated by ATP-want balance btwn purine and pyrimidine synthesis.
- UMP synthase - Orotate phosphoribosyl transferase and Orotidine decarboxylase are separate domains in the same enzyme known as UMP synthase. UMP synthase has 2 activities-synthesizes first real pyrimidine-UMP. OMP is pyrimidine but cannot be used for anything and is just and intermediate

How is UTP formed?
What enzyme is required for CTP synthesis? What is needed for this rxn? What molecule is CTP synthesized from?
UTP is synthesized from UMP by kinases using 2 ATP.
CTP is synthesized from UTP by CTP synthetase using ATP and glutamine.
attached is slide 25 of PP

What is orotic aciduria? What is it caused by?
What are the consequences of this disease?
What is a treatment option for orotic aciduria and how does it help?
Cause: defect in pyrimidine synthesis. Defects in either Orotate phosphoribosyl transferase or orotidine decarboxylase (UMP synthase) result in a build-up of insoluble orotate. Orotate accumulates and circulate which causes aciduria and also precipitates in blood and urine.
Consequences: poor growth, anemia and excretion of orotate in urine At high concentrations orotate forms orange crystals.
Treatment: The anemia and formation of orotate can be diminished by the administration of uridine. (feed back inhibition of orotate synthesis).
What enzymes are required for pyrimidine degradation? What are the final products of pyrimidine degradation?
Nucleases:
- Nucleic acids –> pyrimidine nucleotides
- Phosphatases: nucleotides –> nucleosides
- Nucleoside desaminases:
- Cytidine –> Uridine
- dCytidine –> dUridine
- Uridine phosphorylase:
- Uridine, dUridine –> Uracil;
- dThymidine –> Thymine
- R-1-P -> R-5-P (reused)
Note cytidine turns into uridine so that end products (of this phase) are uracil and thymine
Bases (U and T) are degraded to β-amino acids.
Final products:
U –> β-alanine, NH3, CO2
T –> β-aminoisobutyric acid, NH3, CO2
Note that we have enzymes that can open rings and can excrete products–>no issue with formation of insoluble products as in purine degradation.

How can β-aminoisobutyric acid levels be used clinically?
β-aminoisobutyric acid can be used for diagnostic purposes; high levels are present in cancer patients after chemo- or radiation therapy.
β-aminoisobutyrate is only produced from pyrimidine degradation. can be used to determine efficiency of chemotherapy and titering chemotherapeutic drugs.
Explain the enzymes, substrates, and end products formed in pyrimidine salvage pathways.

What is the starting material for deoxyribonucleotide synthesis? What 2 enzymes are involved in this pathway? What are the products produced?
dNTPs are in low concentrations in non-proliferating cells. Levels rapidly increase during S phase of mitosis or during DNA repair.
Starting material:
ribonucleoside 5’-diphosphates
Enzyme: ribonucleotide reductase (removes hydroxyl group)
Special step: thymidylate synthase. catalyzes fromation of dTMP from dUMP (uracil is not in DNA). this enzyme is important bc (of course) all 4 nucleotides are required for DNA synthesis.

What does thymidylate synthase require for its rxn? What does this do for the enzyme?
- Methylene THF is used to transfer a methyl group to dUMP to form dTMP.
Antitumor agents can slow DNA synthesis and cell division by inhibiting this pathway the dNTP synthesis pathway. What enzymes do the following drugs block?
5-fluorouracil
methotrexate
hydroxyurea
5-Fluorouracil - inhibits thymidylate synthase. if have 3 other nucleotides but not last one (dTTP), do not make DNA.
Methotrexate inhibits the regeneration of THF from DHF (dihydrofolate reductase). THF is needed for dTMP formation (see attached pic).
Hydroxyurea - inhibitor of ribonucleotide reductase. used as a chemotherapeutic agent to block synthesis of deoxyribonucleotides needed for DNA replication effectively blocking cell division.



