Genetics 7 - Epigenetics Flashcards
(54 cards)
learning outcomes

epigenetics
heritable/enduring change in gene expression not related to variation in nucleotide sequence
enduring in the sense of:
the life of a long lived cell
retained from mother to daughter
retained from parent to offspring
epigenetics relate to DNA factors other than nucleotide sequence that impact on gene expression
biochemical basis for epigenetics
Condensation of chromatin and replication of DNA - regulated by chemical modifications of histones - these are influenced by DNA methylation, which occurs in regions of DNA rich in cytosine and guanine and located in promoter regions upstream of genes
DNA methylation - on vs off gene

biallelic expression
both alleles expressed
RECALL
humans are diploid
23 pairs of homologous chromosomes (1 from mother, 1 from father so each have different copies of each gene)
2 copies (often different alleles) of each gene
monoallelic expression
only 1 of 2 alleles is expressed
expression of imprinting
proportion of genes imprinted
involves monoallelic expression
expression of genes in a parent of origin specific manner
only the allele inherited from a specific parent (either maternal or paternal allele) is expressed
< 1% of human genes are imprinted
changes that occur in DNA with imprinting
related to methylation of cytosines (in promoter) and histones
modification of DNA but not nucleotide sequence change
enduring but not permanent change

genomic imprinting

example of imprinting
what allele is expressed
what allele is imprinted
Insulin Like Growth Factor (IGF2)
only paternal allele is normally expressed
maternal allele is imprinted (shut down) - not transcribed or translated into protein - this is to stop too much IGF2 being produced so cell does not grow out of control

if Sally (daughter of Betty and Don) has a child, which allele of IGF2 gene will work
in any oocytes that Sally (mother) makes, whichever version of the allele is present will be switched off
sperm vs ova methylation pattern
imprints are erased in the germline and re-established in gametes
in the sperm all imprints are erased and rewritten with the paternal pattern, even the alleles that came from mum
in the ova all imprints are erased and rewritten with the maternal pattern, even the alleles that came from dad
for imprinted genes, what is required for normal development
expression of imprinted genes
inheritance of maternal and paternal alleles is required
imprinted genes are more vulnerable to mutation - haploid expression
⇒ dominant mutations
Angelman Syndrome symptoms
small
profound intellectual disability
unable to speak and with particular behaviour pattern
happy demeanour with inappropriate laughter
(similar chr area affected as with Prader-Willi)
Prader-Willi Syndrome symptoms
floppy babies - hypotonia
intellectual/cognitive disability
uncontrollable appetite - obesity
hypogonadism in males
incidence approx 1 in 22,000, but uncertainty because PWS may go undiagnosed
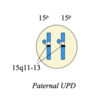
mutation associated with Angelman and Prader Willi
both associated with a de novo deletion on long arm of chr 15 (15q11-q13)
both usually for identical sets of genes but
Prader Willi = deletion from paternal chr 15
Angelman = deletion from maternal chr 15
prader willi deletion
paternal chr 15
angelman deletion
maternal chr 15
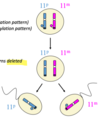
how do deletions (Angelman and Prader Willi) work

Uniparental disomy (UPD)
when 2 copies of a chr come from the same parent
therefore both will have either the maternal or paternal pattern of methylation
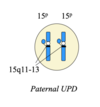
mechanisms of UPD

X-inactivation
heritability
random transcriptional silencing of all but 1 X chromosome in females (maternal or paternal)
heritable from mother cell to all daughter cells in an individual
not heritable from parent to child

when does X inactivation occur
early in embryonic development (totipotent cells) both X chromosomes are active
with differentiation to pluripotent cells random X inactivation (late blastocyst stage)
inactivated X condensed on periphery of nucleus (Barr body)
inactivation of X is retained
through all subsequent mitosis - all daughter cells
but NOT retained across generations