Derm - Skin Cancer Flashcards
What is a melanoma?
malignant tumour arising from melanocytes

What are the worldwide statistics for melanomas?
leads to >75% of skin cancer deaths
rising incident rates observed world wide
What causes the central depigmented zone?

due to tumour regression
What are the main diagnostic tools for skin cancers?
imaging + skin biopsy
Where can melanomas arise?
•Can arise on mucosal surfaces (e.g. oral, conjunctival, vaginal) and within uveal tract of eye
even internal organs can be affected
What are the different categories fo risk factors for melanomas?
- genetic factors
- environmental factors
- phenotypic
What are the genetic risk factors for melanomas?
- Family history (CNKN2A mutations), MC1R variants
- Lightly pigmented skin
- Red hair
- DNA repair defects (e.g. xeroderma pigmentosum)
What are the environmental risk factors for melanomas?
- Intense intermittent sun exposure
- Chronic sun exposure
- Residence in equatorial latitudes
- Sunbeds
- Immunosuppression
What are the phenotypics risk factors for melanomas?
- >100 Melanocytic nevi
- Atypical melanocytic nevi
nevi = proliferations of melanocytes that are in contact with each other, forming small collections of cells known as nests
What pathway is important in melanoma molecular pathogensis? Why?
- Mitogen-activated protein kinase (MAPK) [RAS-RAF-MEK-ERK] pathway
- regulates cellular proliferation, growth and migration
- mutations in this pathway contribute to melanoma growth
What are the different mutations that can lead to melanomas?
- KIT mutation
- NRAS gene
- BRAF gene
- CDKN2A mutations
What do KIT mutations lead to?
- 30-40% of acral and mucosal melanomas
- also melanomas from chronically sun-exposed skin harbour activating mutations or copy number amplifications of KIT gene
What melanomas do mutations in NRAS gene?
15-20% of melanomas
What do mutations in the BRAF gene cause?
- 50-60% of melanomas
- high in melanomas of skin with intermittent UV exposure
- low in melanomas of skin with high cumulative UV exposure
What is the purpose of CDKN2A gene?
- encodes P16 - tumour supressor
- this binds to CDK4/6, preventing formation of cyclin D1-CDK4/6 complex
- Cyclin D1-CDK4/6 complex phosphorylates Rb, inactivating it, leading to E2F release (once released, E2F promotes cell cycle progression)
- therefore prevention of the complex stops cell cycle progression
Why do mutations in the the CDKN2A gene cause melanomas?
can’t encode p16, which stops progression of cell cycle
therefore cell cycle progresses and tumours ariseee
What is the host response to melanomas?
- CD8+ T-cell recognise melanoma-specific antigens and if activated appropriately, are able to kill tumour cells.
- CD4+ helper T-cells and antibodies also play a critical role
- Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) is natural inhibitor of T-cell activation by removing the costimulatory signal (B7 on APC to CD28 on T-Cell)
What immunotherapy is based on the CTLA-4 blokade?
ipilimumab
What are the checkpoint inhibitors in our immune system? What do they do?
PD-1, PDL1
they remove the signal “don’t kill cells” and let immune system kill cancer cells
What is the epidemiology of melanomas?
- Increasing worldwide
- Develops predominantly in Caucasian populations
- Incidence low amongst darkly pigmented populations
- 10-19/100,000 per year in Europe
- 60/100,000 per year in Australia / NZ
What are the different subtypes of melanaomas?
- Superficial spreading
- Nodular
- Lentigo maligna
- Acral lentiginous
- Unclassifiable
What percentage of melanomas are superfical spreading?
60-70%, most common type in fair-skinnned individuals
Where do superficial spreading melanomas usually occur?
- Most frequently seen on trunk of men and legs of women
- Can arise de novo or in pre-existing nevus (without mole or form a pre-existing mole)
What are some characterisitics of superficial spreading melanomas?
- assymetry, border irregularity, colour variation, increased diameter
- In up to 2/3 of tumours, regression (visible as grey, hypo-or depigmentation), reflecting the interaction of host immune system with tumour.

How do superficial spreading melanomas grow?
horizontal growth first (this is where all the visibal echaracteristics show e.g. assymmetry)
then vertical growth (this where you get the appearance of a nodule or bump)
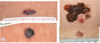
What percentage of melanomas do nodular ones represent?
15%-30% of all melanomas
2nd most common type of melanoma of fair-skinned individuals
Where does it usually appear? What is the demographic?
most commonly trunk, head, neck
more present in M than F
What are the characteristics of a nodular melanoma?
- Usually present as blue to black, but sometimes pink to red, nodule – may be ulcerated, bleeding
- Develops rapidly

What is a nodular melanoma with no pigment called?
amelanotic melanoma
How do nodular melanomas grow?
only has vertical growth phase
therefore the characteristics features of horizontal growth (e.g. assymmetry, border irregularity, etc.) are not present or not obvious)
What percentag eof melanomas do lentigo maligna make up?
minority of cutaneous melanomas (around 10%)
What is the demographic of occurence of lentigo maligna? Where does it commonly occur?
- over 60 year olds
- occurs in chronically sun-damaged skin
- most commonly on the face
What do lentigo maligna melanomas look like?
Slow growing, asymmetric brown to black macule with colour variation and an irregular indented border

What are lentigo malignas usually precursors for?
Invasive Lentigo Maligna Melanoma arises in a precursor lesion termed lentigo maligna
therefore a precursor for invasive melanoma
What percentage of lentigo malignas progress?
5% progress to invasive melanomas
What are some of the characteristics of lentigo maligna under dermatoscope?

What percentage of all melanomas are acral lentiginous?
relatievly uncommon, around 5% of all melanomas
When are acral lentiginous melanaomas usually diagnosed?
most freqeuntly in 7th decade of life
Where do acral lentiginous melanomas typically appear?
palms and soles or in and around the nail apparatus
What is the incidence like across all demgraphics?
- Incidence similar across all racial and ethnic groups
- As more darkly pigmented Africans and Asians do not typically develop sun-related melanomas, ALM represents disproportionate percentage of melanomas diagnosed in Afro- Caribbean (up to 70%) or Asians (up to 45%)
What do acral lentiginous melanomas look like?
a black or brown discoloration that appears on the sole of the foot or palm of the hand. It may resemble a bruise or stain, but over time it grows in size

What do acral lentiginous melanomas look like in the nail? What are the characteristics?
melanonychia = brown-black discoloration of the nail plate and the pigment referred to is conventionally melanin

What is an amenalotic melanoma?

What is key to early detection of melanomas?
self-detection - looking for history of change in colour, shape, or size of a pigmented skin lesion
What does the ABCDE public awareness acronym for self-detection stand for?
A = asymmetry
B = border irregularity
C = colour variation
D = diameter greter than 5mm
E = evolving
What is Garbe’s rule?
If a patient is worried about a single skin lesion, do not ignore their suspicion and have a low threshold for performing a biopsy
What can be some diffrential diagnosis for melanomas?
basal cell carcinoma
seborrhoeic keratosis = harmless skin lesions that increase in number wiht age
dermatofibroma = harmless benign skin lesion
What are poor prognostic features of melanomas?
- Increased Breslow thickness >1mm
- Ulceration
- Age
- Male gender
- Anatomical site – trunk, head, neck
- Lymph node involvement
Stage 1A melanoma have 10 year survival of >95% whereas thick melanomas >4mm and ulceration pT4b have a 10 year survival rate of 50%
How is Breslow thickness measured
measurment hitological from granular layer to bottom of tumour

What invetigative tools are used ot identify melanomas?
dermoscopy
- allows you to see through stratum corneum, negating all the refraction that happens at that level
- allows you to observe features that can’t be seen with the naked eye
What are some global features of melanomas on dermoscopy?
- Asymmetry
- Presence of multiple colours
- Reticular, globular, reticular-globular, homogenous
- Starburst
- Atypical network
- streaks
- atypical dots or globules
- irregular blood vessels
- regression structures
- blue-white veil

How important are dermosocpic lesions?
can improve diagnoses by 50%
but should not be taken in isolation
history + risk factor status are also important
What should be done if there are doubts about the melanoma?
• Excise lesion for histological assessment if in any doubt
IF IN DOUBT, TAKE IT OUT!
How is a melanoma lesion taken out for histological assessment?
• Primary excision down to subcutaneous fat with 2mm peripheral margin

What is done to remove a melanoma?
Wide excision, Margin determined by Breslow depth
- 5mm for in situ
- 10mm for =1mm
Prevents local recurrence or persistent disease
How are melaonomas staged? What is it based on?
- pathology
- TMN

What is a sentinel lymphoma node?
- Lymphatic drainage of finite regions of skin drain specifically to an initial node within a given nodal basin - the ‘sentinel node’
- Represent most likely nodes to contain metastatic disease

When is a sentinel lymph node biopsy offered?
currently offered for pT1b+
When is lymph node dissection done?
when there is Extracapsular spread on lymph node biopsy
What different imaging is done for melanomas and at what stage?
- Stage III, IV and Stage IIc without SLNB
- PET-CT
- MRI Brain
What else is measured in progressing melanomas? Why?
LDH
mjor prognostic factor in metastatic melanomas
How are unresectable + metastatic melanomas treated?
- immunotherapy
- mutated oncogene targeted therapy
What immunotherapy is used to manage melanomas?
• CTLA-4 inhibition – unresectable or metastatic BRAF negative melanoma (Ipilimumab)
• PD-L1 (Programmed cell death ligand) inhibitors (Nivolumab)
Combination immunotherapy not much better than ingle agent in
- Combination immunotherapy leads to 60% response vs 20% monotherapy alone
What mutated oncogene targeted therapy can be used to manage melanomas?
Combination of a BRAF inhibitor (e.g. encorafenib, vemurafenib, dabrafenib) and MEK inhibitor (e.g. trametinib)
Who does keratinocyte dysplasia + carcinomas commonly occur in?
predominantly pale skin types
What causes keratinocyte dysplasia / carcinomas?
solar induced UV damge
What are some different types of keratinocyte dysplasia / carcinoma?
•Actinic keratoses
- Dysplastic keratinocytes
- Bowen’s disease (Squamous cell carcinoma in situ)
- Squamous cell carcinoma
- Potential for metastasis/ death
•Basal cell carcinoma
- (Virtually) never metastasises
- Locally invasive

What contributes to basal cell carcinoma pathogensis?
- UV radiation is significant risk factor
- Dependent on stroma produced by dermal fibroblasts
- Cross talk between tumour cells and mesenchymal cells of stroma
- Receptors for PDGF are upregulated in Stroma but PDGF is upregulated in tumour cells
- BCC has proteolytic activity e.g. metalloproteinases and collagenases – degrade pre-existing dermal tissue and facilitate spread of tumour cells
- Loss of function in chromosome 8q (PTCH gene)
- Sonic Hedgehog-Patched signalling pathway
/ SHH signalling is required for growth of established BCCs
• p53 mutations are also important – majority are missense mutations that carry a UV signature
What contributes to the pathogenesis of squamous cell carcinomas?
- UV radiation is significant risk factor
- Develops through addition of genetic alterations – alterations in p53 are most common, CDKN2A also
- NOTCH1 or NOTCH2 (Wnt / β-catenin signalling) also plays role
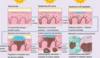
What is the epidemiology of keratinocyte carcinomas?
- Basal cell carcinoma is most common skin cancer
- BCC:SCC 4:1
- Both commoner in pale skin types
- Both more common in men vs women (2-3:1)
- Median age at diagnosis of BCC is 68
What are the risk factors of keratinocyte carcinomas?
•UV exposure
- PUVA
- Fair skin
- Genetic syndromes
- Xeroderma pigmentosum
- Oculocutaneous albinism
- Muir Torre syndrome
- Nevoid basal cell carcinoma syndrome*
- Nevus sebaceous
- Porokeratosis
- Organ transplantation (immunosuppressive drugs)
- Chronic non-healing wounds
- Ionising radiation
- Airline pilots
•Occupational chemical exposures
- Tar, polycyclic aromatic hydrocarbons,
What are actinic keratoses? What are their characterisitics?
- Atypical keratinocytes confined to epidermis
- Erythematous macule or scale or both-> thick papules or hyperkeratosis or both, sometimes cutaneous horn

Where do actinic keratoses usually develop?
develop on sun-damaged skin, usually head, neck, upper trunk or extremities
How do you distinguish actinic keratoses from SCCs?
•Distinction from squamous cell carcinoma sometimes difficult – requiring biopsy
What is the risk of progression for actinic keratoses into SCCs?
0.025–16% per year for any single lesion
What is Bowen’s disease? What are the characteristics?
- squamous cell carcinoma in situ (occupy the skin but haven’t invaded dermis)
- Erythematous scaly patch or slightly elevated plaque
- May arise de novo or from pre-existing AK
- May resemble actinic keratoses, psoriasis, chronic eczema

How are actinic keratoses and Bowen’s disease treated?
- 5-fluorouracil cream =
- Cryotherapy = freezing with liquid nitrogen
- Imiquimod cream = stimulates immune system to attack them
- Photodynamic therapy = apply porforin, sine, light, generate free radicals, cuasing apoptosis
- Curettage and cautery = burning, etc.
- Excision
What do squamous cell carcinomas look like?
- Erythematous to skin coloured
- Papule
- Plaque-like
- Exophytic = describe an abnormal growth that sticks out from the surface of a tissue
- Hyperkeratotic
- Ulceration

What are the high risk features of SCCs?
- Localisation: Trunk and limbs > 2cm; Head / neck > 1cm; Periorificial zones
- Margins: Ill-defined
- Rapidly growing
- Immunosuppressed patients
- Previous radiotherapy or site of chronic inflammation
What histological features of SCCs can identify them as high risk?
- Grade of differentiation: poorly differentiated
- Acantholytic, adenosquamous, demosplasticsubtypes
- Tumour thickness - Clark level: >6mm, Clark IV, V
- Invasion beyond subcutaneous fat
- Perineural, lymphatic or vascular invasion
What is a keratoacanthoma?
- Rapidly enlarging papule that evolves into a sharply circumscribed, crateriform nodule with keratotic core
- Resolves slowly over months to leave atrophic scar
- Controversial entity : Pseudo-malignancy or variant of SCC?

Where do keratoacanthomas normally occur?
most occur on the head or neck, sun-exposed areas
How do you distinguish between keratoacanthomas and SCCs?
Difficult to distinguish clinically and histologically from squamous cell carcinoma
How are SCCs investigated + diagnosed?
- Often clinical diagnosis sufficient
- Diagnostic biopsy may be taken if diagnostic uncertainty
- Ultrasound of regional lymph nodes ± FNA (fine needle aspiration) if concerns regarding regional lymph node metastasis
What are some diffrential diagnoses for SCCs?
basal cell carcinoma
viral wart
merkel cell carcinoma

How are SCCs treated?
- Examination of rest of skin and regional lymph nodes
- Excision
- Radiotherapy
- Cemiplimab for metastatic SCC
- Secondary prevention
When is radiotherapy considered for SCCs?
- Unresectable
- High risk features e.g. perineural invasion
What is a part of secondaey prevention for SCCs?
- skin monitoring advice
- sun protection advice
What are the different subtypes of Basal cell carcinomas?
- nodular
- superficial
- morpheic
- infiltrative
- basisquamous
- micronodular
What percentage of BCCs do nodular ones account for?
most common subtype
accoutns for approx. 50%
What are the cahracteristsics of a nodular BCC?
- Typically presents as shiny, pearly papule or nodule

What are the characteristics of superficial BCCs?
- Well-circumscribed, erythematous, macule / patch or thin papule /plaque
- can resemble Bowen’s disease, actinin keratoses, etc.

What are the characteristics of morphoeic BCC?
- Less common
- Slightly elevated or depressed area of induration
- Usually light-pink to white in colour
- More aggressive behaviour
- Extensive local destruction
What are the histological features of basisquamous BCCs?
Histological features of both basal cell carcinoma and squamous cell carcinoma

What are the characteristsics of micronodular BCCs?
- Resembles nodular basal cell carcinoma clinically
- More destructive behaviour – high rates of recurrence and subclinical spread
How are BCCs investigated + diagnosed?
- Often clinical diagnosis sufficient
- Diagnostic biopsy may be taken
What are the diffrential diagnoses for BCCs?
- SCCs
- Adnexal (Sebaceous) carcinoma
- Merkel cell carcinoma

How do you treat BCCs?
- Standard surgical excision
- Mohs micrographic surgery
When is Mohs micrograph surgery used on BCCs?
- Recurrent basal cell carcinoma
- Aggressive subtype (morpheic / infiltrative / micronodular)
- Critical site
What are some other ways to treat BCCs?
Suitable for superficial BCCs:
- Topical therapy e.g. 5-Fluorouracil, Imiquimod
- Photodynamic therapy
- Curettage
Sutitable for patients over age of 70 due to risks of SCCs:
- Radiotherapy
Suitable for rare BCCs metastases or unresectable BCCs
- Vismodegib - selectively inhibits abnormal signalling in Hedgehog (Hh) pathway
How does micrographic surgery work? Why is this done?
- to preserve tissue that is healthy
- to target the cancerous tissue specifically, rather than missing the tumours in thebread-loafing method or situation
- however, is very time-comsuming, taking hours and hours to resct one tumour that could be done in 45 mins of standard surgery

What percentage of cutaneous lymphomas are T-cell?
75%
What are cutaneous T-cell lymphomas?
Heterogenous group of neoplasms of skin-homing T-cells that show considerable variation in clinical presentation, histological appearance, immunophenotype and prognosis
What are the 2 most common subtypes?
- Sézary syndrome
- mycosis fungoides
What is the underlying molecular pathogensis?
unknown
Inactivation of genes controlling cell cycle and apoptosis has been identified
What is the epidemiology of cutaneous T-cell lymphoma?
- Mycosis fungoides 0.4/100,000
- Typically older adults (median age of diagnosis 55-60)
- Sézary syndrome is rare - <5% of all CTCL
What is mycosis fungoides?
- Common variant of primary CTCL and accounts for 50% of all primary cutaneous lymphoma
- Indolent clinical course

How is a mycosis fungoides lymphoma diagnosed?
• Diagnosis requires skin biopsy
- Diagnosis may take years as skin lesions may be present that are neither clinically nor histologically diagnostic for many years
- lesions may present as eczema or psoriasis for yearss
What can appear as mycosis fungoides?
Atypical T-cell infiltrates which can be found in lymphomatoid drug eruptions
What is the progression of mycosis fungoides?
- Patients progress from patch stage → plaque stage → (finally) tumour stage disease
- Protracted clinical course over years → decades
How can the early stages of MF be characterised?
- Early patch stage is characterised by variably sized erythematous, finely scaling lesions which may be mildly pruritic
- Generally many years of nonspecific eczematous or psoriasiform skin lesions
What is the median duration of onset of skin lesions to diagnosis?
4-6 years, but may vary from several months to more than 5 decades
What si the pathogensis of MF?
- Considered to be a stepwise accumulation of genetic abnormalities → clonal proliferation → malignant transformation → progressive and widely disseminated disease
- Molecular events remain unidentified
- Genetic abnormalities described – not constituting a patter
- P53, CDKN2A, PTEN, STAT3 identified in advanced MF, but not early
e.g. likely secondary genetic events
• Persistent antigenic stimulation plays a crucial role in various lymphomas but no antigens known in MF
How is MF evaluated + staged?
- Type and extent of skin lesions
- Presence of palpable lymph nodes
- Skin biopsies
- Complete blood counts and serum chemistries

What is the treatment for MF?
- Plaque / patch stage treatments include topical corticosteroids, phototherapy and radiotherapy
- Systemic chemotherapy is only indicated in advanced stage when there is nodal or visceral involvement or in patients with rapidly progressive tumours unresponsive to less aggressive therapies
- Brentuximab vedotin (anti-CD30)
What is the prognosis of MF and what does it dpeend on?
- Depends on stage
- 10 year survival rates are:
- > 95% in limited patch / plaque disease
- 85% in generalised patch / plaque disease
- 42% in tumour stage disease
- 20% in those with histological lymph node involvement
What are some diffrential diagnoses for MF?
- psoriasis
- eczema (discoid)
- parapsoriasis

What traid characterises Sezary syndrome?
- Erythroderma
- Generalised lymphadenopathy
- Presence of neoplastic T-cells (Sézary cells) in the skin, lymph nodes and peripheral blood

What is the criteria for Sezary syndrome diagnosis?
- Demonstration of a T-cell clone in peripheral blood by molecular or cytogenetic methods
- Demonstration of immunophenotypical abnormalities an expanded CD4+ T-cell population – resulting in a CD4/ CD8 ratio of greater than 10 and / or aberrant expression of pan-T-cell antigens)
- An absolute Sézary cell count of at least 1000 cells per microlitre
How is Sezary syndrome treated?
- Systemic treatment is required
- Extracorporeal photophoresis = WBCs drained and UV light is used to make them undergo apoptosis
- Chemotherapy can be used
- Skin-directed therapies like PUVA or potent topical corticosteroids may be used as adjuvant therapy
What is PUVA?
combination treatment consisting of taking a drug PSORALEN (P) and then exposing the skin to long-wave ultra- violet light (UVA)
What is Kaposi Sarcoma? What characterises it?
- multifocal systemic disease
- Cutaneous lesions can vary from pink patches to dark violet plaques, nodules or polyps

What causes kaposi sarcoma? What are the risk factors?
HHV8, form of the herpes virus
may be endemic, or related to immunosupression
How is kapsoi sarcoma treated?
chemotherapy (vincristine, doxorubicin, etoposide, bleomycin) and / or radiation is favoured over surgery
What is Merkel cell carcinoma?
Malignant proliferation of highly anaplastic cells which share structural and immunohistochemical features with various neuroectodermally derived cells, including Merkel cells
What are risk factors or causes for merkel cell carcinoma?
- 80% are associated with polyomavirus
- UV exposure is also an aetiological factor
Where do merkel cell carcinomas occur?
•Predilection for the head and neck region of older adults
What are the characteristsics of merkel cell carcinomas?
• Solitary, rapidly growing nodule- pink-red to violaceous, firm, dome shaped,
- Ulceration can occur
• Aggressive, malignant behaviour

What percentage of merkel cell carcinomas develop into advanced disease?
40%
What are the treatments for merkel cell carcinomas?
- Treated with surgery, radiation therapy
- anti-PD1 (Pembrolizumab) / anti-PDL1 (Avelumab)


