Chem I: 11-12 Flashcards
When you add a solution of NaCl to a solution of AgNO3, why is it that the precipitate is AgCl but not NaNO3 in terms of Ksp?
Because AgCl has a lower Ksp value, meaning it is more likely to form a precipitate at a lower concentration.
Which of the following is NOT a salt solubility rule in water?
(A) All group 1 and ammonium salts are soluble
(B) All nitrate, perchlorate and acetate salts are soluble
(C) All carbonate and phosphate salts are soluble
(D) All silver, lead and mercury salts are insoluble, except for their nitrates, perchlorates and acetates
(C) All carbonate and phosphate salts are soluble
Carbonate and phosphate salts are typically INSOLUBLE, unless they are bound to group 1 or ammonium salts.
a) all nitrate, perchlorate, and acetate salts are soluble
b) all carbonate and phosphate salts are soluble
c) all silver, lead and mercury salts are insoluble, except for their nitrates, perchlorates, and acetates
d) all group 1 and ammonium salts are soluble

b) all carbonate and phosphate salts are soluble

solution
homogenous mixtures of 2+ substances that combine to form a single phase
relationship between mixtures and solutions
all solutions are considered mixtures, but not all mixtures are considered solutions
solute
dissolved in a solvent
ex: NaCl, NH3, CO2, glucose
solvent
component of solution that remains in same phase after mixing
if the substances are already in same phase, the solvent is the component present in greater quantity
solvation
aka dissolution
- electrostatic interaction between solute and solvent molecules
- breaking intermolecular interactions between solute and solvent molecules and forming new intermolecular interactions between them
if solvation is exothermic…
process is favored at ___ temperatures
new interactions are stronger than the original ones
low temp
if solvation is endothermic…
process is favored at ___ temperatures
new interactions are weaker than the original ones
high temp –> since new interactions weaker, energy needed to facilitate their formation
ideal solution
when enthalpy of dissolution is 0
spontaneous formation of solutions
exothermic vs endothermic
both can form spontaneously
at constant temp and pressure, entropy always ______ upon dissolution
increases
solubility
max amount of that substance that can be dissolved in a particular solvent at a given temp
saturated
- when max amount of solute has been added –>> dissolved solute is in equilibrium with its undissolved state
- if more solute is added, it will not dissolve
- rates of dissolution and precipitation are equal
dilute
solution in which the proportion of solute to solvent is small
hydration
solvation in water
water molecules break ionic bonds
ions surrounded and stabilized by shell of solvent molecules
hydration rxn
NaCl (s) –>
Na+(aq) + Cl-(aq)
precipitation rxn
ions come together to form a solid that falls out of solution
precipitation rxn
NaCl(aq) + AgNO3(aq) –>
AgCl(s)
concentrated
solution in which the proportion of solute to solvent is small
sparingly soluble salts
solutes that dissolve minimally in the solvent
aqueous solution
solvent is water
H+ is never found alone in solution bc….
a free proton is difficult to isolate
most important solubility rules
all salts of Group I metals and all nitrate salts are soluble
7 general solubility rules
- salts containing ammonium (NH4+) and group I cations are water soluble
- salts containing nitrate (NO3-) and acetate (CH3COO-) anions are water soluble
- halides (excluding fluorides) are water soluble
- exceptions: Ag+, Pb2+
- salts containing sulfate (SO42-) are water soluble
- exceptions: Ca2+, Sr2+, Ba2+, Pb2+
- all metal oxides insoluble
- exceptions: alkali metal, ammonium, CaO, SrO, BaO
- all hydroxides insoluble
- exceptions: alkali metal, ammonium, Ca2+, Sr2+, Ba2+
- all carbonates, phosphates, sulfides, and sulfites are insoluble
- exception: alkali metals and ammonium
complex ion
aka coordination compound
moleculae in which a cation is bonded to at lease one electron pair donor
ligands
electron pair donor molecule in complex ion
coordinate covalent bond
hold complex ions together
electron pair donor and electron pair acceptor from very stable lewis acid-base adducts
complex formation biological applications
- active sites of proteins
- iron cation in hemoglobin
- coenzymes (vitamins) and cofactors
chelation
central cation bonded to same ligand in multiple places
requires large organic ligands that can double back to form a second or third bond with the central cation
concentration
amount of solute dissolved in a solvent
percent composition by mass eq
mass of solute/mass of solution x 100%
mole fraction
eq
XA = moles of A/total moles of all species
sum of mole fractions in a system will always =
1
molarity eq
M = moles of solute/liters of solution
molality eq
m = moles of solute/kg of solvent
true only for dilute aqueous solutions
eq used to determin conc of a solution after dilution
MiVi=MfVf
saturation point
solution concentration is at its max value for the given temp and pressure
when solution is dilute, the thermodynamically favored process is ______.
initially, the rate of ______…..
dissolution
initially, the rate of dissolution will be greater than the rate of precipitation.
as solution becomes more concentrated and approaches saturation, the rate of dissolution ______, while the rate of precipitation _____.
lessens
increases
degree of solubility determined by:
relative changes in enthalpy and entropy associated with the dissolution of the ionic solute at a given temp and pressure
solubility product constant eq
Ksp = [An+]m[Bm-]n
Ksp dependent on
temperature
(and pressure for gases)
As temp increases, Ksp
increases for non gas solutes and decreases for gas solutes
ion product eq
IIP = [An+]m[Bm-]n
difference between ion product and Ksp
concentrations used in IP are concentrations of the ionic constituents at that given moment in time, which may differ from Ksp
IP < Ksp
unsaturated -> soln not yet at equilibirum
solute will continue to dissolve
IP = Ksp
saturated
solution is at equilibrium
IP > Ksp
supersaturated -> solution is beyond equilibrium
precipitation will occur
supersaturated soln
- beyond equilibrium
- thermodynamically unstable
- any disturbance to soln will cause spontaneous precipitation of the excess dissolved solute
molar solubility
molarity of a solute in a saturated soln




every sparingly soluble salt of general formula MX will have Ksp=
Ksp = x2
x: molar solubility (assuming no common ion effect)
every sparingly soluble salt of general formula MX2 will have Ksp=
Ksp = 4x3
x: molar solubility (assuming no common ion effect)
every sparingly soluble salt of general formula MX3 will have Ksp=
Ksp = 27x4
x: molar solubility (assuming no common ion effect)
formation of complex ions _____ the solubility of salt in a soln
increases
formation or stability constant
Kf
for ocmplex ions


common ion effect
presence of common ion results in a reduction in molar solubility of the salte
has no effect on the value of the solubility product constant itself
colligative properties
physical properties of solns that are dependent on the conc of dissolved particles
NOT on the chemical identity of the dissolved particles
include: vapor pressure depression, BP elevations, freezing point depression, osmotic pressure
vapor pressure depression
- raoult’s law
- as solute is added to a solvent, vapor pressure of solvent decrease proportionately
- presence of solute molecules can block the evaporation of solvent molecules, but not their condensation –> reduces vapor pressure
as conc of B increases, vapor pressure of A ______
decreases
as more solute is dissolved into solvent (as more B is dissolved into A), the vapor pressure of the solvent decreases
raoult’s law
eq
PA = XA P°A
PA: vapor pressure of A when solutes present
XA: mole fraction of A
P°A: vapor pressure of A in pure state
raoult’s law hold only when…
the attraction between the molecules of the different components of the mixture is equal to the attraction between the molecules of any one component in its pure state
as vapor pressure decreases, boiling point ______
why?
increases
higher temp is required to match the atmospheric pressure
ideal solutions
solutions that obey raoult’s law


when a nonvolatile solute is dissolved into a solvent to create a soln, the MP of thee soln will be ______ than that of the pure solvent
greater
boiling point
temp at which the vapor pressure of the liquid = the ambient (incident) pressure
extent to which BP of soln is raised
eq
ΔTb = iKbm
ΔTb : inc in BP
i: van’t hoff factor
Kb: proportionality constant (given)
m: molality
van’t hoff factor
number of particles into which a compound dissociates in a solution


freezing point depression
eq
ΔTf = iKfm
ΔTf : freezing point depression
i: van’t hoff factor
Kf: proportionality constant (given)
m: molality
freezing point depends on
concentration of particles, not identity

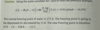
osmotic pressure
amount of pressure that must be applied to counteract the attraction of water molecules for the soln
osmotic pressure
eq
Π = iMRT
Π: osmotic pressure
i: van’t hoff factor
M: molarity
R: ideal gas constant
T: temp
how are molarity and molality related for water?
nearly equal at room temp
only bc 1 L soln is approx = 1 kg solvent for dilute solutions
how are molarity and molality related for solvents besides water?
differ significantly because their densities are not like water
solubility forward reaction
dissolution
solubility reverse reaction
precipitation
solving complex ion problems
- write the normal solubility eq with the salt and the one that makes the complex ions
- if has mole greater than 1 –> 2x not just x
- add them together and multiply the Ks
- ICE
Other equilibrium constants tend to follow the mass-action ratio. Write out this ratio of products and reactants that is equal to Keq or Q (if the reactants and products are not at equilibrium).

Given that the ion product, Qsp, is less than Ksp, which of the following will occur?
(A) More salt will dissolve
(B) No net change in salt dissolving
(C) Less salt will dissolve
(D) Not enough information given
(A) More salt will dissolve
Qsp and Ksp have the same relationship as Q and Keq. If there are less products than the equilibrium suggests should exist (Qsp << Ksp), then more products will form (i.e. salt will dissolve).
Calculate the Ksp of PbCl2 (MM = 278.1) assuming that .14 grams of PbCl2 enters solution upon addition of 14.98 grams of PbCl2 to 147 mL of water?
(A) 3.54 ⋅ 10^-9
(B) 7.98 ⋅ 10^-8
(C) 1.60 ⋅ 10^-7
(D) 3.45 ⋅ 10^-6
(C) 1.60 ⋅ 10^-7
.14 grams PbCl2 ⋅ 1 mol PbCl2 / 278.1 g PbCl2 = approx. 5⋅10^-4 moles PbCl2 (actual: 5.03 ⋅ 10^-4)
5 ⋅ 10^-4 moles / .147 L = approx. 3.5 ⋅ 10^-3 M PbCl2 (actual: 3.42 ⋅ 10^-3)
[Pb2+] = approx. 3.5 ⋅ 10^-3 M [Cl-] = approx. 7 ⋅ 10^-3 M
Ksp = [Pb2+][Cl-]^2 Ksp = (3.5 ⋅ 10^-3)(7 ⋅ 10^-3)^2 Ksp = approx. 1.75 ⋅ 10^-7 (actual: 1.60 ⋅ 10^-7)
Two common methods of defining the amount of solute in a solvent are molarity (M) and molality (m). Write out the definitions (equations) for each.

What is the concentration of Cu2+ at equillibrium upon the addition of Cu(OH)2 (Ksp = 2.2 ⋅ 10^-20)?
(A) 1.8 ⋅ 10^-7 M
(B) 6.9 ⋅ 10^-7 M
(C) 1.8 ⋅ 10^-8 M
(D) 6.9 ⋅ 10^-8 M
(A) 1.8 ⋅ 10^-7 M
Ksp = [Cu2+][OH-]^2
2.2 ⋅ 10^-20 = x * ^(2x)^2
2.2 ⋅ 10^-20 = 4x^3
approx. 5 ⋅ 10^-21 = x^3
x = approx. 2 ⋅ 10^-7 (actual: 1.8 ⋅ 10^-7)
Suppose that a partially soluble salt dissolving is exothermic. According to Le Chatelier’s principle, which of the following would happen if the mixture was heated?
(A) There is no observable change of the salt
(B) There is net dissolving of the salt
(C) There is a net precipitation of the salt
(D) Not enough information is given
(C) There is a net precipitation of the salt
Because heat was added, the system will want to remove heat. If the salt dissolving was exothermic, then the salt precipitating would need to be endothermic and would occur.
PbCl2 (Ksp = 1.60 ⋅ 10^-5) is added to a 8.79 ⋅ 10^-2 M solution of KCl. What is the final concentration of Pb2+?
(A) .00021 M
(B) .0021 M
(C) .021 M
(D) .21 M
(B) .0021 M
Ksp = [Pb2+][Cl-]
1.60 ⋅ 10^-5 = (x)(8.79 ⋅ 10^-2 + 2x)^2 but 2x can be approximated as 0 as compared to the concentration of Cl-:
1.60 ⋅ 10^-5 = (x)(8.79 ⋅ 10^-2)^2
1.60 ⋅ 10^-5 = (x)(approx. 81 ⋅ 10^-4)
(1.6 ⋅ 10^-5)/(8.1 ⋅ 10^-3) = x
x = approx. .2 ⋅ 10^-2 M (actual: .21 ⋅ 10^-2 M)
Does adding H+ to a solution of CaF2 result greater or less solubility of CaF2? Why?
Increased solubility because F- reacts with the H+, causing the solubility reaction to move forward according to Le Chatelier’s principle (removing products).
Does adding NH3 to a solution of AgCl result greater or less solubility of AgCl? Why?
Increased solubility because NH3 reacts with the Ag to form a complex ion, causing the solubility reaction to move forward according to Le Chatelier’s principle.
Solutions are often thought of strictly as involving liquids as the solvent. Which of the following is the general term for a solution of a solid in a solid?
(A) Solenoid
(B) Colloids
(C) Ore
(D) Alloy
(D) Alloy
The majority of elements that are solids at common temperatures are metals. On a side note, ore is more of a mixture because the different compounds can be extracted rather easily, not truly being dissolved.
Which of the following is NOT a colligative property?
(A) Vapor pressure depression
(B) Boiling point depression
(C) Freezing point depression
(D) Osmotic pressure
(B) Boiling point depression
Colligative properties to know are: vapor pressure depression, boiling point ELEVATION, freezing point depression, and osmotic pressure.
Of the following colligative properties, which of the following does NOT have the van’t Hoff factor in its equation?
(A) Vapor pressure depression
(B) Boiling point elevation
(C) Freezing point depression
(D) Osmotic pressure
(A) Vapor pressure depression
Vapor pressure depression is actually modeled by Raoult’s law, which states PA = XA * PAo.
PA = Vapor pressure of solvent A when solutes are present XA = Mole fraction of solvent A PAo = Vapor pressure of solvent A in its pure state
state functions
- describe the physical properties of a system in an equilibrium state
- independent of the process of the system -> how the system got to its current equilibrium
- not independent of other state functions
process function
pathway taken from one equilibrium state to another
ex: work (W) and heat (Q)
state functions mnemonic
When I’m under pressure and feeling dense, all I want to do is watch TV and get HUGS
pressure, density, temp, volume, enthalpy (H), internal energy (U), gibbs free energy, entropy (S)
standard conditions
25 deg C (298 K), 1 atm pressure, 1 M conc
standard temperature and pressure (STP)
0 deg C (273 K), 1 atm pressure
what are standard conditions used for?
kinetics, equilibrium, thermodynamics problems
what is STP used for?
ideal gas calculations
standard state
most stable and prevalent form of a substance under standard conitions
standard states to know
H2(g), H2O(l), NaCl(s), O2(g), C(s)
phase diagrams
graphs that show the standard and nonstandard states of matter for a given substance in an isolated system, as determined by temperatures and pressures
show the temps and pressures at which phases will be in equilibrium
when a substance will be thermodynamically stable in a particular phase

phase changes
solid, liquid, gas
reversible
exist at characteristic temps and pressures
fusion
melting
solid to liquid
occurs at melting point
freezing
crystallization or solidification
liquid to solid
occurs at freezing point
vaporization
evaporation or boiling
liquid to gas
(have enough kinetic energy to leave liquid phase)
condensation
gas to liquid
facilitated by lower temp or higher pressure
evaporation: endo or exothermic?
what is heat source?
endothermicc
heat source is liquid water
each time liquid loses high energy particle, temp of remaining liquid ___inc/dec___
decreases
boiling
- specific type of vaporization
- occurs only under certain conditions
- rapid bubbling of entire liquid with rapid release of liquid as gas particles
boiling vs evaporation
while evaporation can happen in all liquids at all temps, boiling can only occur above the BP of a liquid and involves vaporization through the entire volume of the liquid
vapor pressure increases as temperature _______ because
increases because more molecules have sufficient kinetic energy to escape into the gas phase
vapor pressure
the pressure that gas exerts over liquid at equilibrium
the availability of energy microstates increases as the temperature of the solid __inc/dec__
increases
how do amorphous solids (glass, plastic, chocolate) melt?
melt (or solidify) over larger range of temperatures due to their less ordered molecular structure
sublimation
solid to gas (directly)
deposition
gas to solid
cold finger
may be used to purify a product that is heated under reduced pressure, causing it to sublime
lines of equilibrium / phase boundaries
on a phase diagram
indicate the temp and pressure values for the equilibria

phase diagrams
at which pressure and temp is the gas phase generally found?
high temp, low pressure
phase diagrams
at which pressure and temp is the solid phase generally found?
high temp, high pressure
phase diagrams
at which pressure and temp is the liquid phase generally found?
moderate temp, moderate pressure
triple point
phase digram
point at which the 3 phase boundaries meet
temp and pressure at which the 3 phases exist in equilibrium
critical point
phase diagram
phase boundary between the liquid and gas phases
temp and pressure which there is no distinction between the phases
supercritical fluids
cannot distinguish between the phases
temperatures above the critical point: liquid and gas phases are…
indistinguishable
temperature
(T)
related to the avergae kinetic energy of the particles of a substnce
when a subjects enthalpy increase, its temperature __inc/dec__
increases
heat vs temperature
heat is a specific form of thermal energy transferred between objects as a result of differences in their temperatures
temperature is an indirect measure of a system that looks at the average kinetic energy of particles in a system
heat
(Q)
process function
transfer of energy from one substance to another as a result in differences in temperature
zeroth law of thermodynamics
objects are in thermal equilibrium only when their temperatures are equal
first law of thermodynamics eq
ΔU = Q - W
U: internal energy
endothermic
processes in which the system absorbs heat from surroundings
endothermic
eq ΔQ
ΔQ > 0
exothermic
processes in which the system releases heat into surroundings
exothermic
eq ΔQ
ΔQ < 0
enthalpy
ΔH
equivalent to heat (Q) under constant pressure
calorimetry
process of measuring transferred heat
heat (q) absorbed or released in a given process
eq
calorimetry
q = mcΔT
c: specific heat of substance
ΔT: change in temp
specific heat
amount of energy to raise the temperature of one gram of a substance by one degree C or K
specific heat of H2O (l)
c = 1 cal/gK
heat capacity
mass x specific heat
bomb calorimeter
aka decomposition vessel
constant volume
isolated system
bomb calorimeter
eqs
no heat is exchange between calorimeter and rest of universe
ΔUsystem = -ΔUsurroundings
qsystem = -qsurrounds
qcold = -qhot
phase change rxns and temp
phase change reactions do not undergo changes in temperature
cannot use q = mcΔT
enthalpy/heat of fusion (ΔHfus)
used when
used to determine the heat transferred during phase change between solid-liquid
when transitioning from solid to liquid, change in enthalpy is….
positive because heat must be added
when transitioning from liquid to solid, the change in enthalpy is…
negative because heat must be removed
which eq to use
liquid gas boundary
enthalpy of vaporization (ΔHvap)
q = mL
L: latent heat
latent heat
enthalpy of isothermal process
specific heat vs heat capacity
specific heat (c) is energy required to raise the temp of one gram of a substance by 1 degree celsius
heat capacity (mc) is the product of mass and specific heat
constant volume vs constant pressure calorimetry
constant volume - as reaction proceeds, the temp of the contents is measured to determine the heat
constant volume - heat measured indirectly by assessing temp change in water bath around the vessel
endothermic eq
ΔHrxn
ΔHrxn > 0
exothermic eq
ΔHrxn
ΔHrxn < 0
standard enthalpy of formation
(ΔH°f)
enthalpy required to produce one mole of a compound from its elements in their standard states
ΔH°f of element in standard state =
0
standard enthalpy of rxn eq
ΔH°rxn = ΣΔH°f,products - ΣΔH°f,reactants
hess’s law
enthalpy changes of reactions are additive
applies to any stat function, including entropy and free energy
bond enthalpy
avg energy that is required to break a particular type of bond between atoms in gas phase
endothermic processes
bond breakage is __endo/exothermic___
endothermic
positive H
bond enthalpy eq
ΔH°rxn = ΣΔHbonds broken - ΣΔHbonds formed = total energy absorbed - total energy released
standard heat of combustion
ΔH°comb
enthalpy change associated with the combustion of a fuel
the larger the alkane reactant, the __more/less__ numerous the combustion products
more
second law of thermodynamics
energy spontaneously disperses from being localized to becoming spread out if it is not hindered from doing so
entropy
measure of spontaneous dispersal of energy at specific temperatures
how much energy is spread out, or how widely spread out energy becomes
change in entropy eq
ΔS = Qrev/T
Qrev: heat that is gained or lost in a reversible process
entropy units
J/molK
when energy is distributed into a system at a given temperature, its entropy __inc/dec__.
increases
when energy is distributed out of a system at a given temperature, its entropy __inc/dec__.
decreases
ΔSuniverse =
ΔSsystem + ΔSsurroundings > 0
gibbs free energy
measure of the change in the enthalpy and the change in entropy as a system undergoes a prcoess
indicates whether a reaction is spontaneous or not
change in free energy
max amount of energy released by a process – occurring at constant temp and pressure – that is available to perform useful work
gibbs free energy eq
ΔG = ΔH - TΔS
Goldfish are Horrible without (minus sign) Tartar Sauce
TΔS represents
total amount of energy that is absorbed by a system when its entropy increases reversibly
spontanous rxn eq
ΔG < 0
decrease in free energy
exergonic
nonspontaneous rxn eq
ΔG > 0
movement away from equilibrium position
endergonic
exergonic
spontaneous rxn
ΔG < 0

endergonic
nonspontaneous rxn
ΔG > 0

ΔG = 0
system is in equilibrium
ΔG < 0
spontaneous
ΔG > 0
nonspontaneous
ΔG is temperature dependent when
ΔH and ΔS have the same sign
ΔH > 0
ΔS > 0
outcome
spontaneous at high T

ΔH > 0
ΔS < 0
outcome
nonspontaneous at all T
ΔH < 0
ΔS > 0
outcome
spontaneous at all T
ΔH < 0
ΔS < 0
outcome
spontaneous at low T
ΔGºrxn =
ΔGºrxn = -RTlnKeq
ΔGrxn =
ΔGrxn = ΔGºrxn + RTlnQ = RTln(Q/Keq)
Q = reaction quotient
if Q/Keq < 1
reaction proceeds spontaneously forward
if Q/Keq > 1
reaction proceeds un reverse direction spontaneously
if Q/Keq = 1
reaction at equilibrium
open system
can exchange both energy and matter with environment
closed system
no exchange of matter with environment
internal energy
U
sum of all the different interactions between and within atoms in a system
ΔU in closed system =
ΔU = Q - W
modified standard state
[H+] = 10-7 M
pH = 7
reactions with more products than reactants have a more __neg/pos__ ΔG
negative
reactions with more reactants than products have a more __neg/pos__ ΔG
positive
why can heat be used as a measure of internal energy in living systems?
cellular environment has a relatively fixed volume and pressure, which eliminates work from our calculations of internal energy
all spontaneous reactions are
irreversible
What is the thermodynamic quantity that combines enthalpy and entropy? What is its units?
Gibbs free energy (ΔG) is the thermodynamic quantity that combines enthalpy and entropy. Its units are typically kJ/mol.
Why is Gibbs free energy being a state function important in calculating ΔG and if a series of reactions is favorable?
Gibbs free energy being a state function means that ΔG values for different reactions can be added together to see if the overall reaction is favorable or not. It allows for coupling of reactions.
Would the formation of the product be favored or not if the ΔG value is positive?
The formation of the product would not be favored if delta G is positive. A positive ΔG means the reaction would require huge amounts of energy to form the product.
Suppose that a reaction has ΔH= -77 kJ and ΔS= -0.48 kJ. At what temperature will it change from spontaneous to non-spontaneous?
(A) 47 K
(B) 160 K
(C) 243 K
(D) 321 K
(B) 160 K
ΔG = ΔH - TΔS 0 = (-77) - T(-0.48) 77 = 0.48T T = about 150K (actual 160.4K)
At 160 Kelvin, this reaction will change from spontaneous to non-spontaneous. Because this transition is when ΔG = 0, if we substitute in the equation that ΔG = 0, we could solve for T.
What is the difference between heat, temperature, and enthalpy?
Heat is the transfer of energy due to change in temperature.
Temperature is the measure of the average kinetic energy of molecules.
Enthalpy is referred to as the heat transfer from the perspective of the system during reactions.
True or false? When looking up enthalpy values for a set of reaction species, the enthalpy depends on the concentration of the species.
True. The enthalpy depends on the concentration of the species.
When 1.0 mole of ZnO(s) decomposes, the ΔH = 348 kJ/mol of heat energy. This tells you that the formation of ZnO(s) is:
(A) Endothermic
(B) Exothermic
(C) In equilibrium
(D) Endergonic
(B) Exothermic
The FORMATION of ZnO(s) is exothermic because it is the reverse reaction of the decomposing.
What is the change in enthalpy for the following reaction: 2Mg + O2 -> 2MgO, if ΔH Mg = 0 kJ, ΔH O2 = 0 kJ, and ΔH MgO = -501 kJ/mol?
(A) 1,002 kJ
(B) 501 kJ
(C) -501 kJ
(D) -1,002 kJ
(D) -1,002 kJ
The change in enthalpy is:
(ΔH products) - (ΔH reactants)
Thus the answer is -1,002 kJ.
The laws of thermodynamics dictate transformations of energy from one form to another. Which law of thermodynamics states that the total amount of energy in the universe is constant?
(A) Zeroth law of thermodynamics
(B) First law of thermodynamics
(C) Second law of thermodynamics
(D) Third law of thermodynamics
(B) First law of thermodynamics
The first law of thermodynamics states that the total amount of energy in the universe is constant.
The laws of thermodynamics dictate transformations of energy from one form to another. Which law of thermodynamics states that the entropy of a system approaches some constant value as its temperature approaches absolute zero?
(A) Zeroth law of thermodynamics
(B) First law of thermodynamics
(C) Second law of thermodynamics
(D) Third law of thermodynamics
(D) Third law of thermodynamics
The third law of thermodynamics states that the entropy of a system approaches some constant value as its temperature approaches absolute zero.
True or false? Hess’s Law states that the energy change of a process is independent of the path that was taken to get there.
True. Hess’s Law states that the energy change of a process is independent of the path that was taken to get there.
Hess’s Law is true for variables such as enthalpy and entropy because these are _________ variables.
(A) Process
(B) State
(C) Fixed
(D) Variable
(B) State
Hess’s Law is true for variables such as enthalpy and entropy because these are state variables.
At a constant pressure, the change in enthalpy is equal to what?
(A) The temperature change
(B) The heat added to the system
(C) The molarity change
(D) The disorder added to the system
(B) The heat added to the system
At a constant pressure, the change in enthalpy is equal to the heat added to the system.
True or false? A reaction can be both exergonic and endothermic.
True. A reaction can be both exergonic (spontaneous) and endothermic (requiring heat). However, these reactions are quite rare, and must have a high increase in entropy. A common example would be an instant icepack!
What is a reversible process vs. an irreversible process?
A reversible process is one in which a reaction can go forwards and backwards without losing any energy, right near equilibrium in an ideal world.
An irreversible process is one in which a reaction can’t go forwards and backwards without losing any energy.
When the pressure on a gaseous reaction system at equilibrium is increased, the equilibrium will shift in which direction according to Le Chatlier’s principle?
The equilibrium will favor the side with the least gas molecules.
For the following reaction, how will the reaction equilibrium be affected by an increase in temperature? H2O2(l) -> H2(g) + O2(g), delta H = 187 kJ
Since delta H is a positive value it indicates that it is an endothermic reaction and the energy is on the reactant side because it is being absorbed. Thus an increase in the temperature of the system causes equilibrium to shift towards the right, towards the products H2(g) and O2(g) because this causes the reaction to shift away from the heat to balance out the reaction.
For the following reaction, how will the reaction equilibrium be affected by an increase in volume?
H2O2(l) = H2(g) + O2(g)
The reaction will shift to the right, thus increasing the products. H2O2 is a liquid, so it cannot change its volume. H2 (g) and O2 (g) can both take advantage of the increased volume by expanding and spreading out the molecules. Thus an increase in H2O2 will shift the reaction to the right towards H2 (g) and O2 (g).
If volume is decreased for the following reaction system at equilibrium, what color will you expect the mixture to become?
N2O4(g) (colorless) + heat = 2 NO2(g) (pink)
You would expect the mixture to become more colorless because there are less gas molecules on that side of the equation. Shifting the equilibrium to the side with less gas molecules will relieve the stress of decreasing volume (which inherently means increasing pressure).
a) ores are mixtures, whereas alloys are solutions
b) ores are found only below sea level and are mined for, whereas alloys are found above sea level
c) ores are solutions found in nature, whereas alloys are man made solutions
d) ores strictly describe metals that are trapped in igneous rock, and are a subclass of alloys

a) ores are mixtures, whereas alloys are solutions

the reaction quotient, Q, tends to change so it approaches the equilibrium constant, K. which of the following statements is not true when Qsp > Ksp?
a) A common laboratory technique to purify compounds is to allow Q to be greater than K, followed by extracting that desired salt while the impurities remain soluble.
b) This can be caused by saturating a solution while the solvent is chilled, followed by letting the solution heat up.
c) Q can approach K by having some of the salt recrystallize, or precipitate out
d) This can be caused by a solution having some of its solvent evaporate
a) A common laboratory technique to purify compounds is to allow Q to be greater than K, followed by extracting that desired salt while the impurities remain soluble

a) 1, with NaCl having the greater vant hoff factor
b) 3, with K2SO4 having the greater vant hoff factor
c) 1, with K2SO4 having the greater vant hoff factor
d) 5, with K2SO4 having the greater vant hoff factor

c) 1, with K2SO4 having the greater vant hoff factor

a) [Ag+] is greater for pure water soon; [Cl-] is greater for NH3 soln
b) [Ag+] and [Cl-] are greater for NH3 soln
c) [Ag+] and [Cl-] are greater for pure water soln
d) [Cl-] is greater for the pure water soln; [Ag+] is greater for NH3 soln

a) [Ag+] is greater for pure water soon; [Cl-] is greater for NH3 soln

a) decrease the temp
b) add CaCl2
c) add HCl
d) stir the soln

c) add HCl

a) neither copper (II) carbonate nor cobalt (II) carbonate will precipitate
b) copper (II) carbonate will precipitate
c) cobalt (II) carbonate will precipitate
d) both copper (II) carbonate and cobalt (II) carbonate will precipitate

c) cobalt (II) carbonate will precipitate

a) 1.8 x 10^05 g/L
b) 2.1 x 10^-10 g/L
c) 1 x 10^10 g/L
d) 9 x 10^-6 g/L

a) 1.8 x 10^05 g/L

a) 2.3 x10^-5
b) 1.1 x 10^-5
c) 8 x 10^-8
d) 1.6 x 10^-7

d) 1.6 x 10^-7

a) III only
b) I and II only
c) I, II, and III
d) I and III only

b) I and II only

a) he did not account for the air pressure in the lab
b) he did not account for the conc of the compounds used in lab
c) he had no theoretical mistakes, and physical mistakes probably caused these
d) he did not account for stoichiometry in enthalpy calc

d) he did not account for stoichiometry in enthalpy calc

a) switching to a larger container
b) adding catalyst
c) adding inert gas
d) adding CaCO3

a) switching to a larger container


d


b


d


c


a


d


b


b


b


d


b



