Week 5: Parasitology Part I Flashcards
(145 cards)
learning objectives
For the parasitic organisms identify
- the geographic region of risk
- mode of transmission
- Life cycle
- Clinical symptoms
- Clinical complications of infection
- Laboratory diagnostic tests
- Appropriate treatment
- side effects of treatment
Case 1


Describe the distribution of Malaria

Question

Malaria is transmitted by the bite of the female Anopheles mosquito
Describe the mechanism of transmission of Malaria
- Malaria is transmitted by the bite of the female Anopheles Mosquito
- Sporozoites in the mosquito’s saliva are injected into the human bloodstream
- Vector-borne infection

Question

Merozoites
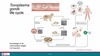
Describe the life-cycle of Malaria

Malaria is caused by?
Plasmodium species
Plasmodium characteristics
- Obligate intracellular protozoa
- Single-celled Eukaryotic
- The zygote is the only diploid stage in Plasmodium life-cycle
Tropism of Plasmodium
Blood protozoa with hepatic stage
Diploid stages of Plasmodium
The zygote is the only diploid stage in life-cycle
Definitive host of Plasmodium
Mosquito (sexual reproduction in the gut of the mosquito)
Intermediate host of Plasmodium
Human
(Asexual reproduction in liver and blood stages)
Plasmodium type and location of reproduction in definitive host
Mosquito (Sexual reproduction in the gut of the mosquito)
Plasmodium type and location of reproduction in intermediate host
Human (Asexual reproduction in liver and blood stages)
Factors associated with the pathogenicity of Plasmodium
4 listed
- Penetration of anatomic barrier via mosquito bite
- Avoidance of immune detection
- Antigenic variation, molecular mimicry, intracellular location, suppression of parasite-specific B & T-cell responses
- Replication in the host
- Endotoxin in P. falciparum
How do Plasmodium avoid immune detection
- Antigenic variation
- molecular mimicry
- Intracellular location
- suppression of parasite-specific B & T-cell responses
Question

D. Thick & thin peripheral blood smear
Thick and thin peripheral blood smear

Clinical symptoms of Malaria are caused by?
blood-stage parasites and the host immune response
Pathophysiology of Malaria infection
- RBC destruction
- Intravascular hemolysis -> severe microcytic, hypochromic anemia
- Cytokine release
- Schizont rupture -> macrophage stimulated to release TNF and IL-1 cytokines
- Sequestration of infected RBCs
- Adhere to capillary endothelial cells -> impair blood flow
- Splenomegaly
- Biochemical & electrolyte changes
- Hypoglycemia due to parasite glucose consumption, decreased hepatic gluconeogenesis, quinine causing pancreatic insulin release
- Metabolic acidosis from microvascular ischemia, parasite lactate production
- Hyponatremia

Pathophysiology of Malaria as a result of RBC destruction
Intravascular hemolysis -> severe microcytic hypochromic anemia
Pathophysiology of Malaria as a result of cytokine release
Schizont rupture -> macrophage stimulated to release TNF and IL-1 (cytokines)
Pathophysiology of Malaria as a result of sequestration of infected RBCs
- adhere to capillary endothelial cells -> impair blood flow
- Splenomegaly