Exam 1: Healing and Repair Flashcards
Goal of Healing
- Restore tissue architecture and function
- Repair parenchymal tissues
- Heal the surface
Repair Processes
-
Regeneration
- Cells that survive injury proliferate
- Sometimes added by stem cells
- Need cells that can proliferate
- Need intact stroma & basement membrane
- More normal/functional result
- Cells that survive injury proliferate
-
Scar formation ⇒ CT deposition
- Occurs if
- Tissue incapable of complete restitution
- Supporting structures severely damanged
- Not normal but can provide enough stability to allow some function
- Occurs if

Proliferative Capacity
3 groups:
- Labile cells
- Stable cells
- Permanent cells
Labile Cells
- Continuously dividing tissues
- Multiply throughout life
- Can regenerate as long as stem cells preserved
- Ex:
- Epithelial surface cells
- Lymphoid/hematopoietic cells
- Epithelium of GI/GU tract
Stable Cells
“Quiescent cells”
- Latent capacity to regenerate
- Includes:
- Parenchymal cells of kidney, liver, pancreas
- Fibroblasts
- Smooth muscle
- Endothelial cells
- Lymphocytes
Permanent Cells
- Non-dividing tissues
- Do not divide enough to regnerate
- Includes:
- Neurons
- Cardiac muscle
- Skeletal muscle
- Satellite cells can help
Cell Proliferation
Driven by many growth factors:
- Activated macrophages most important source
- Cells use integrins to bind ECM proteins
- Activate signaling pathways
- Induce protein production ⇒ drives cell through cell cycle
- Release blocks on cell cycle
Tissue Regeneration
Labile Cells
- Tissue stem cells normal quiescent until needed
- Due to soluble factors, cell-cell, and cell-ECM interactions
- Most are not totipotent
- Limited types of differentiated cells they can generate
- Injury stimulates cells to divide and differentiate
Tissue Regeneration
Stable Cells
Ex. Liver:
- Loss of liver tissue from infection, injury, surgery
- Can adequately regenerate even if 90% is lost
- Phases:
-
Priming phase
- Kuppfer cells make cytokines
- Makes hepatocytes competent to receive & respond to growth factors
-
Growth factor phase
- Growth factors drive primed hepatocytes into cell cycle
- Then non-parenchymal cells proliferate
- Endothelial, Kuppfer, Stellate
-
Termination Phase
- Heptocytes exit cell cycle & return to quiescence
-
Priming phase
-
Progenitor cells can also be activated
- Found in Canals of Hering

Scar Formation
Causes
- Regeneration not enough for repair
- Severe or chronic tissue injury
- Damage to parenchymal cells, epithelial, CT frame
- Injury of non-dividing cells
Scar Formation
Process
- 3 key steps:
- Angiogenesis
- Deposition of CT ⇒ formation of granulation tissue
- Remodeling of CT
- Macrophages important
- Clear microbes/dead tissue
- Provide growth factors
- Secrete cytokines
- Repair begins within 24 hours of injury
- See granulation tissue in 3-5 days
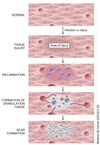
Scar Formation
Timeline

Scar Formation
Area Preparation
- Immune system removes offending agent and inflammatory exudate
- Lysosomal enzymes liquefy debris
- Inflammatory response ends
- Fibroblasts recruited to begin remodeling
Scar Formation
Angiogenesis
Formation of blood vessels:
-
Changes in hemodynamics
- NO ⇒ vasodilation
-
Vascular endothelial growth factor (VEGF) ⇒ ↑ vascular permeability / promote angiogenesis
- Contributes to edema in healing wounds
-
Pericytes seperate from adluminal surface
- Breaks down basement membrane
- Allows formation of vessel sprout
-
Endothelial cells
- Migrate towards injury
- Proliferate behind leading front (“tip”) of migrating cells
- Remodel into capillary beds
-
Periendothelial cells recruited to form mature vessels
- Pericytes for capillaries
- Smooth muscle cells for larger vessels
- Endothelial proliferation & migration suppressed
- Basement membrane deposited

Scar Formation
Granulation Tissue Formation
- Highly vascularized CT composed of:
- New capillaries
- Infiltrating and proliferating fibroblasts
- Produces loose CT
- Pink/red and granular in gross appaerance
- Occupies tissue defect until scar can mature

Scar Formation
Collagen Deposition
- Two steps:
- Fibroblasts migrate and proliferate into site of injury
- Fibroblasts produce and deposit ECM proteins
- Controlled by local cytokines
- Mostly from M2 macrophages
- TCF-β most important
- Mostly from M2 macrophages
- Further ECM deposition ⇒ more distance between capillaries and fibroblasts
- Less erythema
- Some fibroblasts become myofibroblasts

Scar Formation
CT Remodeling
- Need balance between synthesis and degradation of ECM proteins
- Done through remodeling
-
Matrix Metalloproteinases (MMPs)
-
Degrade collagen and other ECM components
- Collagenases, gelatinases, stromelysins
- Produced by many cell types
- Must be activated by proteases at site of injury
- Inhibited by specific Tissue Inhibitors of Matalloproteinases (TIMPs) made by mesenchymal cells
-
Degrade collagen and other ECM components
Healing
Influencing Factors
-
Infection
- Prolongs inflammation & ↑ local injury ⇒ delays healing
- DM
-
Glucocorticoids
- Anti-inflammatory
- ⊗ TGF-β production ⇒ weakens scar, ↓ fibrosis
-
Nutritional status
- Especially protein & Vit C deficiency
-
Mechanical factors
- ↑ local pressure or torsion ⇒ dehiscence
-
Perfusion
- Arteriosclerosis, DM
- Obstructed venous drainage e.g. varicose veins
- Foreign body
-
Tissue type
- Only stable and labile cells capable of restoration
-
Extent of injury
- Need intact parenchyma/BM/stem cells
-
Location of injury
- Inflammation in tissue spaces ⇒ extensive exudates
- Pleural, peritoneal, synovial
- Exudates must be digested/reabsorbed by leukocytes
- Can restore as long as there is not cellular necrosis
- Large exudate can organize ⇒ granulation ⇒ scar
- Inflammation in tissue spaces ⇒ extensive exudates
Collagen Deposition
Effects of Nutrition
- Type III collagen deposited initially in granulation tissue
- Type III replaced by Type I collagen
- Hydroxylation of lysine and proline needed to crosslink
- Vit C required
- Cu2+ is a co-factor for lysyl hydroxylase
-
Collagenase required for transition from Type III ⇒ Type I
- Zn2+ is a co-factor
- Hydroxylation of lysine and proline needed to crosslink
First Intention Healing
Overview
- Occurs through primary union
- Wound edges are apposed
- Primary mech ⇒ epithelial regeneration
- Limited # of dead cells
- Only minor discontinuites in BM
First Intention Healing
Process
-
Day 1
- Formation of fibrin clot ⇒ coagulation
- Neutrophils arrive within 24 hours
-
Day 2
- Epithelial cells from both edges migrate and proliferate along edges
- Deposition of BM components
- Thin epithelial layer closes the wound
-
Day 3
- Neutrophils replaced by macrophages
- Wound debrided
- Fibroblasts arrive
- Start collagen production
- Epidermis covering wound now near normal thickness
- Neutrophils replaced by macrophages
-
Day 5
- Peak of neovascularization
- Granulation tissue filling incisional space
- New vessels are leaky
-
Week 2
- Fibroblast proliferation and collagen deposition continues
- See fewer neutrophils, vessels, and edema
-
End of 1st month
- Scar is cellular CT
- Nearly no inflammatory cells
- Covered by nearly normal epidermis
- Dermal appendages in line of incision are lost

Second Intention Healing
- Occurs when wound edges are not in contact ⇒ tissue lost
- Repair through a combo of regeneration and scarring
- Characteristics
- Slower
- Larger fibrin clot
- More exudate and necrotic debris
- More intense inflammatory reaction
- More granulation tissue
- Accumulation of ECM and larger scar
-
Wound contraction can occur due to myofibroblasts
- ↓ size of large skin defects to 5-10% of original by 6 weeks
- More complications

Wound Strength
-
7 days s/p wound
- 10% tensile strength
- Low collagen content
-
60-70 days s/p wound
- 30% tensile strength
- 100% collagen content
- High ratio of Type III : Type I collagen
-
3 months s/p wound
- 70-80% tensile strength
- 100% collagen content w/ remodeling and turnover
- Inc. Type I : type III collagen ⇒ 85% of normal skin Type I
- Never reaches 100% of tensile strength
Fibrosis
Overview
Abnormal deposition of collagen in internal organs due to chronic disease.
-
Caused by persistent injurious stimuli
- Chronic infections and/or immunological reactions ⇒ loss of tissue ⇒ attempt at repair
-
Develops in space occupied by inflammatory exudate
- Ex. Organizing pneumonia
- Results in organ dysfunction and/or organ failure
-
TGF-β ⇒ major cytokine involved
- Collagen made by myofibroblasts in lung & kidney
- Collagen made by stellate cells in liver







