Molavi Chapter 8 - Colon and Appendix Flashcards
(38 cards)
Can you diagnose tubular adenoma if the endoscopist did not see a polyp?
NO
The history and gross anatomy matters
Where should you vs should you not see Paneth cells
You should see Paneth cells in the ascending and transverse colon
You should NOT see Paneth cells in the descending colon.

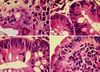
Colonic tubular adenoma
Stand out at low power by looking blue.
Crypts are depleted of goblet cells and mucin and have cigar-shaped and/or pseudostratified hyperchromatic nuclei.
Dysplasia must extend all the way to the surface epithelium to qualify as an adenoma. If there are signs of maturation, it is more likely a reactive change.
“Tubular” adenoma
Smooth surface, parallel crypts, similar to the normal epithelium but with full-thickness low-grade dysplasia.
“Villous” adenoma
Covered in finger-like papillary projections

“Tubulovillous” adenoma
Has features of tubular and villous adenomas


Tubular adenoma with high grade dysplasia
Effectively equivalent to carcinoma in-situ.
Diagnosis made on the basis of architecture and cytology. Glands become cribriform, fused, or back-to-back. Cytologic features include total loss of nuclear polarity, significant pleomorphism, atypical mitoses, and large nucleoli.
Often for cases where “you are worried about carcinoma but can’t quite find any areas of invasion”
To diagnose invasive carcinoma in the colon, you must demonstrate cancer . . .
. . . crossing the basal lamina into the lamina propria
Clues to invasion include jagged interface with the lamina propria, individual infiltrating cells, desmoplastic response, and “pinking up” of the invasive cells

Invasive colonic adenocarcinoma
Note the poorly-formed glands clearly witihn the lamina propria.

Hyperplastic polyps
The surface shows a characteristic “frilly” appearance with hyperplastic mucinous epithelium. Deeper crypts how star-shaped (serrated) lumens.

Sessile serrated adenoma
Associated with the microsatellite instability cancer pathway.
Have a characteristic widening and horizontal branching at the base (“duck feet”, arrow). Epithelial cells may be more eosinophilic (less mucin) and pseudostratified than a typical hyperplastic polyp. The surface looks similar to a hyperplastic polyp though.
Clinically, these are treated as adenomas – not as hyperplastic polyps.

Inflammatory pseudopolyp
Polypoid structure consisting of granulation tissue or inflamed lamina propria with distorted crypts.
When adjacent to an ulcer, there may be significant reactive changes ressembling dysplasia – but surface maturation should still be visible.
Three pathways to adenocarcinoma in the colon
-
APC or “chromosome instability” pathway:
- APC is inactivated ⇒ KRAS and/or BRAF mutation ⇒ p53 mutation ⇒ carcinoma
-
“Microsatellite instability” pathway
- Mismatch repair mutations in areas of repeated sequences (microsatellites) ⇒ instability of microsatellite regions ⇒ carcinoma
-
CpG island methylator phenotype (CIMP) or “epigenetic instability” pathway
- Methylation of promoters of tumor suppressors or MMR genes ⇒ convergence with MSI or APC pathways ⇒ carcinoma
Colon tumor features that predict high microsatellite instability
- Arising from the right sided colon
- Mucinous, medullary, or signet-ring variants
- Prominent lymphocytic infiltrate

Medullary carcinoma of the colon
Distinct and rare variant associated with dense lymphocytic reaction, but a bland, almost neuroendocrine cytology.
When seen, medullary carcinoma suggests Lynch syndrome or a sporadic MSI-type tumor.
MSH2, MSH6, MLH1, PMS2
Mutations of the HNPCC (hereditary non-polyposis colon cancer) syndrome (specifically the Lynch syndrome subtype) family mutations. These mutations are associated with a high risk of MSI-type colon cancers (medullary, mucinous, signet ring) as well as endometrial cancer, ovarian cancer, and prostate cancer.
MSH = Mismatch Repair (involved in mismatch repair)
MLH = MutL Homolog (pairs with PMS2 to form a dimeric protein involved in regulating DNA repair response)
PMS = Postmeiotic segregation (pairs with MLH to form a dimeric protein involved in regulating DNA repair response)

The HNPCC umbrella


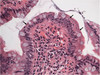
Well-differentiated neuroendocrine tumor (Carcinoid tumor)
The most common locations for a GI neuroendocrine tumor are the appendix and small bowel. They are often submucosal, and often present as SBO.
Well-differentiated NETs are characterized by a uniform, neuroendocrine-type cytology and trabecular, spindly, rosette-like architecture separated by ribbons of fibrovascular septa. Rarely they may appear to be pseudo-gland forming, so be doubly careful that you are not looking at a NET if you have an unusually bland and homogenous adenocarcinoma on your hands.

Low-grade appendiceal mucinous neoplasm
Tumors with bland or low-grade cytology, may extravasate mucinous epithelium (or just mucin) into the wall of the appendix all the way up to the serosa. The extent of mucin spread determines the tumor stage even in the absence of stromal invasion. May compress the lamina propria basically out of existence, as seen here.

High-grade appendiceal mucinous neoplasm
Mucinous tumors with high-grade dysplasia, but lacking usual colonic-type stromal invasion and desmoplastic response.
Like LAMN, may extravasate mucinous epithelium (or just mucin) into the wall of the appendix all the way up to the serosa. The extent of mucin spread determines the tumor stage even in the absence of stromal invasion.

Appendiceal mucinous adenocarcinoma
Note the full invasion of the stroma and fibrous, desmoplastic response.
Pseudomyxoma peritonei
When a mucinous tumor literally fills the peritoneum with mucus.
Histologically this disease is classified by the tumor cell producing it.
If there are no free-floating carcioma cells, it may be described as “acellular mucin.” But, if there is even a single identifiable epithelial cell, it is a mucinous carcinoma peritonei. It is described as low- or high-grade depending upon the degree of atypia.

Active colitis
Neutrophils are seen at the epithelium of the crypts (cryptitis) and there is an ulcerated surface epithelium (arrowhead).
Cryptitis vs crypt abscess
Cryptitis: Neutrophils in the crypt epithelium
Crypt abscess: Neutrophils in the crypt lumen