Molavi Chapter 24 - Thyroid and Parathyroid Flashcards
(48 cards)
Two basic cell types of the thyroid
Follicular epithelium (TTF-1, PAX8, thyroglobulin positive)
C cells (TTF-1, neuroendocrine marker, and calcitonin positive, thyroglobulin negative)
The only well to histologically differentiate a follicular adenoma from follicular carcinoma is. . .
. . . to examine the entire capsule margin
Follicular carcinomas can look just like adenomas
Hurthle cell adenoma
Adenoma of thyroid follicular cells displaying Hurthle cell change
Cells are large pink oncocytes with round nuclei which may be enlarged or slightly irregular. Nucleoli may be prominent.

Follicular thyroid carcinoma
- Since it can appear histologically identifical to adenomas, it is defined by the presence of EITHER: capsular OR vascular invasion (note that vascular invasion within the capsule does count).
- Dominated by a microfollicular pattern
- Said to be “poorly differentiated” if there is high mitotic rate and necrosis. Architecture may also be lost at this grade.

Poorly differentiated follicular thyroid carcinoma
Instead of follicles the tumor is more ribbon, cord, or slit-like. The grade is also higher.

Papillary thyroid cancer
- Classic Orphan Annie eye nuclei with peripheral chromatin – an articat produced by formalin fixation and not seen on frozen section or cytology. Stands out as glassy or pale on low-power
- Crowded, overlapping, pleomorphic nuclei, sometimes with coffee-bean grooves and pseudoinclusions (invaginations of cytoplasm)
- Overall papillary architecture
- Psamomma bodies may be present. Typically only seen when there is papillary arthitecture.
- Variants:
- Papillary microcarcinoma
- Follicular variant papillary thyroid carcinoma
- Noninvasive follicular neuplasm with papillary-like nuclear features (NIFTP)
- Diffuse sclerosing
- Tall cell

NIFTP algorithm

NIFTP vs FVPTC
NIFTP = Noninvasive follicular thyroid neoplasm with papillary-like nuclear features; benign
FVPTC = Follicular variant of papillary thyroid carcinoma; malignant
- The key to differentiating:
- If it is unencapsulated and infiltrative, it is FVPTC that behaves like papillary carcinoma and is signed out as such
- If it is encapsulated and invades the capsule or vasculature, it is FVPTC that behaves like follicular carcinoma and is signed out as such
- If it is surrounded by an intact capsule and behaves in a benign fashion, it is signed out as NIFTP

Thyroid papillary microcarcinoma
Papillary carcinoma variant
Histologically identical to papillary carcinoma, but by definition <1 cm and incidentally discovered.
If solitary, they are considered benign.
If they are clinically discovered, they fail to meet this definition and are considered papillary carcinoma, regardless of size.
Diffuse sclerosing papillary carcinoma
Rare, but important to recognize due to worse prognosis.
Widely infiltrative as opposed to discrete/mass forming.
Characteristics: desmoplastic or sclerotic stroma, squamous metaplasia, tons of psammoma bodies, dense lymphocytic infiltrate, extensive lymphovascular invasion
Anaplastic thyroid carcinoma
Aggressive tumor that arises from de-differentiation of papillary or follicular carcinoma.
Appear as “sheets” of pleomorphic cells. At this grade they have usually lost their TTF-1 and thyroglobulin expression. However, they retain PAX8 staining.

___ is a common mimicer of papillary carcinoma
Hashimoto’s thyroiditis is a common mimicer of papillary carcinoma
It an sometimes have Orphan Annie-like nuclei due to a fixation artifact. A carcinoma arising from Hashimoto’s should stand out sharply from its neighbors.
Hyalinizing trabecular tumor
Has textbook papillary nuclei, including the grooves and inclusions, but an architecture similar to medullary carcinoma: Well-circumscribed nodules of nests and cords (trabecular) set in a dense, pink, hyalinized stroma.
This is a benign lesion.
Differences in thyroid carcinoma spread
Follicular: Spreads locally.
Papillary: Spreads through lymph nodes.
Medullary thyroid carcinoma
Salt-and-pepper nucleated cells that grow in nests.
May look like anaplstic on low power, but nuclear features and IHC can differentiate it reliably.

Spindle Epithelial Tumor with Thymus-like Elements (SETTLE)
Thought to arise from entrapped ectopic thymus tissue within the thyroid, like CASTLE.
Has a biphasic pattern: spindle cells and epithelioid structures (cords, tubules, papillae, glandular formation). No or rare lymphocytes. Mitotic activity and focal necrosis are rare.

Carcinoma showing thymus-like elements
Thought to arise from entrapped ectopic thymus tissue within the thyroid, like SETTLE.
Fibrous bands separating islands/nests of cells. Variable lymphocytes and plasma cells.
Tumor cells have ill-defined borders, vesicular nuclei, prominent nucleoli, OR may show squamous differentiation with distinct cell borders and eosinophilic cytoplasm.

Oncocytic change is due to . . .
. . . accumulation of altered mitochondria in the cytoplasm.
Calcium oxalate and the thyroid
Calcium oxalate is present in normal thyroid tissue in the cholloid mixture
It can help differentiate thyroid from parathyroid in borderline cases.
Remember, calcium oxalate is best seen under the polarizer.
Classical triad of Hashiomoto’s thyroiditis
- Hurthle cell change
- Lymphocytes (often forming follicles/germinal centers)
- Plasma cells

Thyroglossal duct cyst
Anterior midline cyst
Lined by respiratory or squamous epithelium.
In many cases, there will be a small amount of thyroid tissue within the wall of the cyst.
Often resection and specimen will include the hyoid bone, because there may be thyroid tissue within the hyoid itself.
Ultimobranchial body cell nest / solid cell nest
Microscopic developmental remnant of the ultimobranchial body
The ultimobranchial stage of thyroid development includes both follicular and parafollicular cells.
Lining may be squamous, respiratory, or transitional.
Elongated, grooved nuclei with squamoid features. p40+ p63+. It is important to recognize these and not confuse them with microcarcinoma, and to know that C cells may be among them.

Lymphoepithelial cyst
Squamous epithelium with lymphoid stroma
May be a branchial cleft cyst within the thyroid

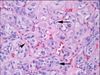
What’s this white stuff?

Calcium oxalate crystals
This is a sure way to identify thyroid tissue on frozen section.
Otherwise, parathyroid with pseudofollicles can be an incredibly convincing mimic on frozen.

















