3.10 - 3.12 Cell to cell communication and signalling Flashcards
What is the difference between contact dependent or paracrine signalling?
- This is for short distance
- Contact dependent is where cells are in close contact, membrane to membrane
- paracrine is where there is an extracellular release of signal that acts only locally on neighbouring cells

What are the long distance types of cell signalling?
- Synaptic where neurons have an electrical signal along the axon (long distance) resulting in release of neurotransmitter across the synapse (short distance)
- Endocrine where there is a release of hormone into the bloodstream which acts widely throughout the body

What type of signalling occurs at a very short distance?
- Autocrine where cells can stimulate themselves if they have receptor for the ligand
- Groups of identical signalling cells (community) can reinforce signals, for example in development it ensures cells follow the same differentiation pathway
- Cancer cells use autocrine signals to stimulate their own survival and proliferation

How can signalling occur in a gap junction?
- Allows direct communication between cytoplasm of adjacent cells by small intracellular signalling molecules such as Ca2+ or cAMP
- Allows neighbouring cells to coordinate responses to signal such as noradrenaline response in liver cells Cx32

What are the two types of receptors?
- Cell surface receptors for a hydrophilic ligand that cannot cross the membrane. For example peptide growth factors bind cell surface receptor
- Intracellular for a hydrophobic or lipophillic ligand which can cross the membrane, for example steroids or small molecules diffuse across the membrane, bind intracellular/nuclear receptors

What is the basic cell signalling pathway?

How does the same signal (acetylcholine) cause different responses in different cells?
- Due to different receptor types
- Muscarinic (G protein) vs Nicotinic (ion channel) receptors
- Different intracellular mediators

What are the characteristics of steroid hormones?
- They are transported in blood by carrier proteins (steroid-hydrophobic)
- Cross plasma membrane
- Bind intracellular receptors that have DNA binding domains (receptors are homo or heterodimers)

How are intracellular receptors activated?
- Receptors kept inactive as they have inhibitory proteins bound to them
- When ligand goes into cytosol it kicks off inhibitory protein, binds to the pocket and induces conformational changes



What are the 3 major classes of cell surface receptors?
- Ion channel couple receptors
- G-protein couple receptors (indirectly linked to enzymes)
- Enzyme coupled receptors

What are the two important classes of enzyme - coupled receptors?
- Receptor tyrosine kinases – have kinase activity and phosphorylate ‘Tyr’ on intracellular signal proteins
- Receptor serine/threonine kinases – have kinase activity and phosphorylate ‘Ser’ and ‘Thre’ on target proteins
What are the two classes of ligands for receptor tyrosine kinases?
Secreted growth factors and hormone
- Epidermal growth factor (EGF)
- Fibroblast growth factor (FGF)
- Platelet-derived growth factors (PDGF)
- Hepatocyte growth factor (HGF)
- Insulin, Insulin-like growth factor (IGF)
- Vascular endothelial growth factor (VEGF)
- Macrophage colony stimulating factor (M-CSF) • Neurotrophin (eg. nerve growth factor NGF)
Membrane-bound ligands
• Ephrins
What are the domains like for receptor tyrosine kinases?
- Single transmembrane domain
- Highly variable extracellular domains
- Similar intracellular domains (tyrosine kinase domains)

How do ephrins/eph receptors function together?
- very large family
- Can function in bidirectional signalling
- Ligand can signal back to the cell, main signal is via phosphorylation of Eph receptors, but ephrins often linked to the cytoskeleton so the signalling cell can receive a response as well
- Often functions in cell migration and axon guidance (attraction or repulsion cues)

What is the signalling pathway for eph?
- Ephrins causes clustering of the eph receptors. Receptor on the migrating cell engages with ephrins and dimerises due to cross phosphorylation on a specific tyrosine
- A kinase binds resulting in phosphorylation of ephexin (GEF)
- Ephexin activates RhoA
- RhoA results in myosin-actin interactions and growth cone collapse
- No gene transcription, very rapid response
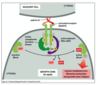
What conformational change do receptor tyrosine kinases undergo when a ligand binds?
- Ligand (dimer or multimer) binding causes receptors to dimerise (unlike G protein coupled receptors)
- Dimerisation causes cross phosphorylation of each receptor (autophosphorylation)

What does the phosphorylated receptor tyrosine kinase bind?
- The phosphorylated (activated) receptor binds other intracellular proteins via phospho-tyrosines
- Enzymes such as phospholipase Cgamma, phosphatidylinositol-3’-kinase, Src
- Docking proteins such as Grb2 which act as intermediary for enzyme to bind

What are Src Homology domains?
- Binding proteins have homologous phsopho-tyrosin binding domains
- SH2 binds activated phospho-tyrosines on receptor
- SH3 binds domains in other intracellular proteins
- Where that protein sits depends on its folding but once folded the SH3 domain interact with phosphotyrosine

What is the SH2 domain of the binding protein for receptor tyrosine kinase?
- it has two binding pockets, one for phosphotyrosine and one for amino acid side chain which is usually adjacent to phosphorylated tyrosine
- Phosphotyrosine same shape no matter what
- Plug and socket

How is Ras activated from receptor tyrosine kinase signalling?
- Activated RTK binds SH2 domain of Grb-2
- Grb-2 = docking protein (via SH3 domain) for guanine nucleotide exchange factors (GEFs) – eg Sos.
- SOS (GEF) activates Ras by exchange of GDP for GTP
- Ras now binds GTP and activates molecules downstream

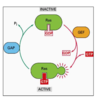
How does Ras function as an ON/OFF switch?
- Superfamily of monomeric GTPases such as Ran, Rab
- In the inactive state it binds GDP
- Activated by guanine exchange factors (GEFs) such as sos, where it exchanges GDP for GTP
- GTPase activating proteins (GAPs) result in increased hydrolysis of GTP
- Hyperactive Ras mutants are resistant to GAP which leads to cancer
Is the receptor tyrosine kinase and Ras active indefinitely?
- No receptor tyrosine kinase and Ras are active only for very short periods
- Action of phosphatases on receptors and GAPs (GTPase activating proteins) on Ras
- Therefore need signalling pathway to rapidly propagate these signals for proliferation and differentiation
What is happening in this experiment?

- This experiment shows how short the activation of Ras is
- Ras is manipulated genetically and expressed in cells
- Linked GTP to a red fluorescent dye. When red comes in close contact with yellow there is resonance energy transfer
- When Ras binds GTP two fluoro molecules come close and you can measure the fluorescence coming off the red GTP
- Ras only active for 4-5 minutes

What cascade does Ras activate?
- Ras activates downstream Ser/Thr kinase cascade (MAP kinase)
- Serine/threonine-PO4 longer lived than tyrosine-PO4
- MAPK (Erk) has both Thr and Tyr which ensure activation is specific, only by MAPKK (Mek)
- Acive Erk enters the nucleus and activates multiple gene regulatory proteins (eg G1 cyclins)
- MAPKK (Mek) is activated by MAPKKK (Raf)

How does the MAPK pathway stimulate proliferation?
- Ras activates the MAPK cascade
- MAPK (Erk) leads to expression of immediate early response genes such as Myc, Fos, Jun
- Myc activates the cell cycle through various mechanisms
- Immediate early response genes are transcribed very quickly and in the absence of protein synthesis

What does Myc do from the MAPK pathway?
- Myc activates expression of delayed response genes including cyclin proteins that act in G1 of cell cycles
- D cyclins bind and activate cdk proteins (G1 phase cdk)

What does active G1-cdk go on to do in the MAPK pathway?
- Actuve cyclin/cdk complex phosphorylates and inactivated Rb protein
- Rb normally binds and keeps E2F protein inactive
- Phospho-RB dissociates from E2F
- Active E2F activates transcription of cell cycle genes (S phase cyclins)
- Rb tumour suppressor gene
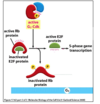
What are the feedback loops in the MAPK pathway?
- S phase cyclins cause DNA synthesis and entry into S phase
- feedback loops in place so that Rb remains phosphorylated
- E2F positive feedback onto itself

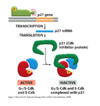
What happens during cell cycle arrest to the G1/S-Cdk?
- Active p53 activates transcription of a CDKI (p21)
- p21 binds and inactivates the G1 Cdk/cyclin complex so there is no progression into S phase
- Failure to correct DNA damage results in accumulation of mutations (cancer)
What happens if cell cycle is overactivated?
- Hyperactivated Ras may cause excessive Myc
- Myc duplications cause excessive Myc
- Cells often display cell cycle arrest or death
- Excess myc activity leads to increased arf expression
- Arf inactivates the mdm/p53 complex leading to cell cycle arrest or cell death
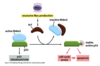
What is the superfamily for receptor serine/threonine kinases?

Are the receptor serine/threonine kinases always active?
- Ligands are often inactive/latent, pro-peptide, binding proteins, ECM
- Activated by acidic conditions, inflammation, enzymes (MMPs), integrins
What are the roles of receptor serine/threonine kinases in the TGFB superfamily?
- Important regulators of cell processes in development and adult tissues
- Pattern formation in the embryo
- Tissue specification
- Extracellular matrix production
- Wound healing, fibrosis
- Cell death, anti-proliferative/ proliferative (tissue type dependent)
- Epithelial-mesenchymal transition (EMT).
What are the two major types of receptor serine/threonine kinases?
- Type I receptors (ALK1-8)
- Type II receptors - specific for each type of ligand (Act RIIA, ActRIIB, TBRIIs, BMPRII) but can be promiscuous with type I receptors
- Ligand induces tetramerisation where type II phosphorylates Type 1
- Smad proteins recruited to phosphorylated type I receptor
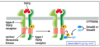
What are the 4 different types of smads?
- Smads are transcription factors that illicit the responses
- Receptor smads include smads 2,3 which mediate activin/TGFB signals and smads 1,5,8 which mediate BMP singlas
- Common smad includes smads 4 which binds Receptor-smads and activates transcription - SBE
- Inhibitory smad included smads 6,7 which inhibits Receptor-smads from being phosphorylated or the trimeric complex from going in to the nucleus
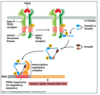
What happens when TGFbeta receptors are activated?

- Activated TGFB receptors are endocytosed via clathrin coated pits (receptor mediated endocytosis)
- Most of the signalling occurs in early endosomes (SARA protein facilitates Smad docking and phosphorylation)
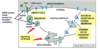
How does TGFB induce EMT?
- Represses epithelial genes (Eg. Adherens junction proteins)
- Activates mesenchymal genes (Eg. ECM proteins, Intermediate filaments)
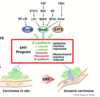
What happens to cells in epithelial mesenchymal transition?

What is shown in this experiment?

- Constituitionally active human TGFB1 cDNA does not have the propeotide so it is always active
- Trans gene inserted into early embryo of mice
- The pink line is the basal membrane
- In trans gene lots of ECM produced, huge proliferation and change in phenotype
- Change phenotype of epithelial cells by regulating TGFB signalling
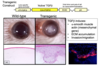
How is EMT a feature of human cataracts?
- Anterior subcapsular cataract is a plaque of transformed epithelial cells which have excessive abnormal extracellular matrix (collagen I/III, fibronectin)
- Myofibroblasts have loss of epithelial characteristics
- Mesenchymal markers have alpha-smooth muscle actin
- Associated with inflammatory diseases of the eye and physical damage with high incidence in korea

What are ion linked channel receptors?
- Ionotropic
- Where ligand binds directly to ion channel receptor, no second messengers



What is the structure of G proteins?
- They have three subunits alpha beta gamma, of which alpha and gamma are membrane tethered
- Various types of G protein specific for groups of GPCR
- Inactive state has alpha subunit GDP bound and active state alpha subunit GTP bound

How does the G protein conformation change when the ligand binds?
- Ligand binding gives conformational change in GPCR so G protein can bind GPCR
- GPCR acts as a guanine exchange factor (GEF)
- Active GPCR causes release of GDP and binding of GTP
- GTP causes conformational change in G protein and activates alpha subunit and the beta,gamma subunit

What happen in G protein signalling when the alpha subunit is activated?
- Once activated the alpha subunit (+GTP) binds to a target protein and activates it
- The alpha subunit is alpha GTPase which causes hydrolysis of GTP to GDP
- GTPase activity of alpha subunit is enhanced by binding to target or RGS proteins (Regulator of G protein signalling or GAP protein for Ras inactivation?

How does the G protein get switch off?
- GTP-GDP hydrolysis causes dissociation of alpha subunit from the target protein - switch OFF
- Hydrolysis of GTP to GDP inactivates the alpha subunit and it reforms inactive G protein with beta,gamma subunits

How does G-protein-receptor signalling target cAMP?
- Common target for G proteins is adenylyl cyclase which catalyses ATP to cAMP
- Cyclic AMP has many targets and affects many processes
- cAMP binds PKA regulatory subunits
- PKA dissociates from regulatory subunits and becomes activated

What is an example of a toxin targetting cAMP production?
Cholera toxin overactives G protein which affects the Cl- channel and causes diarrhoea
In G protein receptor signalling what does active PKA go on to do?
Active PKA enters nucleus and activates gene transcription by phosphorylating a transcription factor (CREB), which binds associated binding protein (CBP)



How does the beta,gamma subunit of the G protein cause signaling?
- Activated beta,gamma subunits can also bind to a target protein and activate them
- In the heart muscle acetylcholine can bind G-protein linked receptor. Activated beta,gamma subunit binds to a K+ channel and opens it
- Loss of K+ ions out of cell decreases contraction and decreases the heart rate
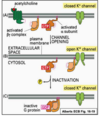
What sort of rapid signalling pathways are G-protein linked receptors involved in?
- Smell receptors activated by food and stimulation of saliva
- Adrenaline stimulation of heart rate
- Fastest is the response of photoreceptors to light (~20 ms)
- Achieved by rhodopsin (G- protein coupled light receptor) – affects cGMP-gated Na+ channel
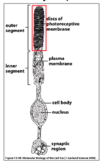
What is the pathway for phototransduction in rods?
- Rhodopsin (G coupled light receptor) is linked to cis-retinal
- Light-induced isomerisation of cis-retinal (cis to trans) causes conformational change in rhodopsin
- Transducin (G-protein) alpha subunit activates cGMP phosphodiesterase
- Drop in cGMP closes cGMP gated Na+ channels causing hyperpolarisation
- Hyperpolarisation causes calcium channels to close and low calcium reduces glutamate release

How is the light signal switched off in phototransduction?
- RGS (GAP) protein binds to transducin (G protein) which hydrolyses GTP to GDP
- Rhodopsin kinase phosphorylates rhodopsin which inhibits rhodopsin activation
- Arrestin binds phospho-rhodopsin and further inhibits activity
- Low calcium stimulates Gulanylate cyclase which stimulates cGMP production

How are bipolar cells in the retina excited or inactivated?
- In the dark photoreceptors release glutamate to inhibit ON bipolar cells and excite OFF bipolar cells
- In the light, photoreceptor hyperpolarisation stops inhibition of ON and inactivates OFF bipolar cells
- Activated bipolar cells synapse and transmit signals to ganglion cells and then to the brain.



Where does the Wnt ligand come from?
- 19 different Wnt ligands identified in mammals
- Highly glycosylated
- Originally identified in Drosophila (Wingless, Wg).
- Int gene found in mice – common integration site for mammary tumour virus (MMV).
- Wg + Int = Wnt
What are the 3 different pathways that Wnt proteins can activate?
Canonical:
- Wnt/B-catenin – relies on regulating degradation of B-catenin protein
Non-canonical:
- Ca2+ pathway
- Planar cell polarity (PCP) pathway – Rho GTPases, PCP proteins
Where is B-catenin released to at adherens junctions?
- Adherens Junctions in epithelial cells are dynamic
- AJ reassembly results in B- catenin release in cytoplasm
- Cell needs to recycle OR get rid of cytoplasmic B-catenin (Proteolysis)
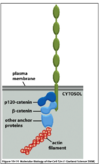
What does the B-catenin destruction complex comprise of?
- Complex of cytoplasmic complex of proteins target B- catenin for ubiquitylation and degradation by proteasomal enzymes
- Includes Axin, glycogen synthase Kinase 3B (GSK3B), Casein Kinase 1 (CK1), Adenomatous polyposis coli (APC)
- CK1 and GSK3B phosphorylate B-catenin on phospho Ser/Thr residues, which are targets for E3 ubiquitin ligase complex

What is the Wnt/B-catenin signalling pathway with and without Wnt signal?

How does B catenin control stem cell differentiation in hair follicles?
- Knockout of B-Catenin inactivates Wnt pathway.
- Inactivation of Wnt pathway in the hair follicle cells of skin inhibits stem cell adopting a follicle fate but adopt an epidermal fate instead.
- No hair follicles form (loss of stem cell proliferation)
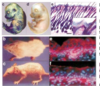
How does this show that Wnt signal controls lens epithelial cell fate?
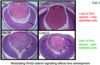
- When you knockout B catenin those stem cells cannot replciate and do not produce more epithelial cells
- If you knockout APC10 there is no longer a funcitonal complex that can degrade B catenin so B catenin levels rise, increasing expression of myc and producing lots of proliferating cells
- CatX10 is where B-catenin is mutated so that it cannot be phosphorylated
- Remove one exon which contained all the phosphorylation sites, still produces functional b catenin that functions in adhesion junctions but when it gets into destruction complex it can’t be phosphorylated
- Protein of B catenin can’t be phosphorylated or degraded

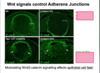

How does over active Wnt cause proliferation?
- APC is a tumour suppressor which controls cell cycle by controlling beta-catenin
- Mutations in APc or Catnnb (B-catenin) cause activation of Wnt pathway
- Wnt signals regualte Myc and cyclin D expression causing G1/S phase transition
- Wnt pathways mutations cause over proliferation

How does overactive Wnt lead to cell death?
- In lenses with mutations of the wnt pathway there is increased cell death
- Activation of p53 means cellc cannot arrest the cell cycle because of myc
- p53 causes apoptosis

How are mutations in the Wnt-pathway (APC) associated with colon polyps?
- LOH of Apc or oncogenic B-catenin causes increased proliferation (stem cells)
- Failure to differentiate (fate switch) to form polyps
- At this stage still not malignate cancer need the other hallmarks of cancer



