1.4 Lipids and Carbohydrates Flashcards
What are the three broad functions of lipids in biology?
- Storage
- Structural
- Signals
What specific lipids are the storage lipids?
Fatty acids
Triacylglycerol
Waxes
What specific lipids are the membrane lipids?
Phospholipids
Glycolipids
Cholesterol
What specific lipids are the signalling and cofactor lipids?
- Phospholipid derivatives (inositol phospholipids)
- Steroid hormones (cholesterol derivatives)
- Eicosanoids (paracrine hormones eg. prostaglandins)
- Lipid soluble vitamins (vitamin A)
What defines a fatty acid?
Carboxylic acids with hydrocarbon chains containing between 4 to 36 carbons. Usually even number of carbons and unbranched naturally
Types of fatty acids


What is the trend seen with increasing length of the carbon backbone of fatty acids for melting point and solubility in water?
Melting point increases and solubility decreases

How are saturated fatty acids able to pack more closely?
- The fully saturated C backbone is usually in a fully extended conformation
- Saturated fatty acids can therefore pack into a nearly crystalline array, stabilised by extensive hydrophobic interactions of the hydrocarbon chain
How are the dobule bonds usually found in unsaturated fatty acids?
The double bond is usually in the cis form and is usually NOT conjugated
How is the double bond affected by unsaturated fatty acids?
- Unsaturated cis fatty acids pack less orderly due to the kink
- Less extensive favourable interactions
- It takes less thermal energy to disrupt disordered packing of unsaturated fatty acids so they have a lower melting point







What is the difference between simple and mixed fatty acids?
Simple = all three fatty acids are identical (triolein, tripalmitin)
Mixed = fatty acids differ
Why are fatty acids advantageous as storage lipids?
- Higher energy yield than oxidation of other fuel sources such as glycogen or starch
- Not hydrated so they weigh less




When drawing a model of the fluid mosaic model what components should you include?
- Glycolipid
- Oligosaccharide
- GPI anchored protein
- Sterol
- Integral protein
- Phospholipid
- Sphingolipid
- Peripheral protein
- Peripheral protein covalently linked to lipid
How can phospholipids be further classed?
Glycerophospholipids are based on the glycerol molecule
Sphingolipids are based on sphingosine
Each has a polar head group and two non polar tails

What is the most common glycerophospholipid and its structure?
Phosphatidylcholine

What is the simplest example of a glycerophospholipid?
Phosphatidic acid where X = H



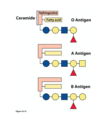
What is the simplest example of a sphingolipid?
Ceramide where X of the alcohol = H

How else can the structure of sphingolipids be changed apart from this?