Cell membrane Flashcards
Transporters: hydrophobic molecules
- O2, CO2, N2, benzene
- flow effortlessly across lipid bylayer

Transporters: small uncharged polar molecules
- H2O, urea, glycerol
- not as efforless, but can still cross the lipid bylayer easily

Transporters: Large uncharged polar molecules
- glucose, sucrose
- too big and hydrophilic to cross, require channel
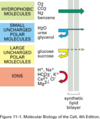
Transporters: Ions
- H+, Na+, HCO3+, etc
- unable to cross at all without channel

permeability water vs glucose
water is 105 times more permeable than glucose
Plasma solute concentrations
- Na: 140
- K: 4.3
- Cl: 105
- HCO3-: 24
- phosphate: 2
- Protein: 1
- Total Osm: 291
- pH: 7.4
ECF solute concentrations
- Na: 140
- K: 4.3
- Cl: 105
- HCO3-: 24
- phosphate: 2
- protein: 0
- Total Osm: 290
- pH: 7.4
ICF solute concentrations
- Na: 12
- K: 120
- Cl: 5
- HCO3-: 12
- Phosphate: 100
- protein: high
- Total osm: 290
- pH: 7.2
uniport
- channel for one solute going one direction
symport
channel for two different solutes going in same direction
ex. Na bringing glucose into capillaries from intestine
antiport
channel for two different solutes going in opposite directions
Nernst Equation
used to calculate cell potential
Veq = (61/Z) * log (Kout/Kin) mV
membrane potential
The voltage caused by the difference in concentration of ions on different sides of a cell membrane
depolarization
the interior voltage of a cell becoming less negative
repolarization
the interior of a cell becoming more negative
action potential
when the membrane potential of a cell rapidly rises and falls
*for an action potential to happen, the membrane potential must depolarize to the critical threshold
steps in action potential
- Trigger raises membrane potential over threshold
- Na+ channels open, Na comes in, membrane potential rises
- Na channels close, K channels open
- K leaves cells, membrane potential falls, below resting
- K channels close, leaving only “leak” channels

Michaelis Menten equation
V= (Vmax * S)/km+S
S= substrate concentration
V = rate of reaction (enzyme productivity)
km = constant
Vmax = constant
Km
The Michaelis Menten constant which shows the concentration of the substrate when the reaction velocity is equal to one half of the maximal velocity of the reaction.
primary active transport
energy is used to bring one molecule through a channel agains the concentration gradient
Ex. 1 ATP brings 3 Na out of cell
secondary active transport
energy is used to move something against concentration gradient, but when that same thing gets back into cell, it brings another molecule.
Ex. one ATP pushes 3 Na out of cell, when Na goes back in, it brings Glucose in. In this way, energy is only used for one of the channel crossings, glucose gets in free

aquaporins
helps to transfer water across membranes when it needs to be moved more quickly.
Ex. in kidneys
ligand
a molecule that produces a signal by binding to a receptor site on a target protein
G-Protein coupled receptors
cAMP
- ligand connects to receptor and activates G-protein
- G-protein made up of Alpha, beta, and gamma
- GDP is released from the G- protein and replaced by GTP
- G protein activates Adenylyl (adenylate) cyclase.
- The activated Adenylate Cyclase then catalyzes ATP to become cAMP, the secondary messenger which activates the effector protein Protein Kinase-A
- Protein Kinase A phosphorlates specific proteins and causes desired effect




