April 29, 2016 - Proton Handling Flashcards
The Kidney and Acid-Base Balance
To reabsorb buffer (HCO3 and citrate)
To secrete H+ (and generate HCO3-)
Bicarbonate Reabsorption in the PCT Cell
NHE3 brings sodium into the cell from the lumen in exchange for hydrogen going out. This is a major source of sodium reabsorption in the kidney.
Hydrogen in the lumen combines with bicarbonate through the help of carbonic anhydrase to create CO2 and H2O.
CO2 moves along its concentration gradient into the cell, where it meets with H2O and carbonic anhydrase to create bicarbonate and H.
Bicarbonate is sent back into the blood through a co-transporter with sodium.
Hydrogen is sent back through NHE3 back out of the cell in a cycle, while more sodium is brought in.

Failure to Reabsorb Bicarbonate
A problem with NHE3, carbonic anhydrase, or the sodium-bicarbonate cotransporter cause proximal (Type 2) renal tubular acidosis (RTA).

Titratable Acid Excretion
The more acid we have the more we can get rid of due to the pK (dissociation constant) of HPO4 / H2PO4.
HPO4 can accept another H at low pH’s. However, this system is fixed and does not allow us to adapt to heavy loads of acid.
Glutaminase
Glutaminase activity is pH-dependent. It becomes more active in acidosis and less active in alkalosis.
Producing NH4+ is the best way of getting rid of acid.
The distal nephron secretes hydrogen and chloride, but hydrochloric acid would be too strong. Ammonium is pumped into the interstitium where it reaches a high concentration. It then meets hydrochloric acid in the cortical collecting duct where it forms ammonium chloride which is a weak acid.
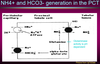
The Role of NH4+ in H+ Excretion
Ammonia reaches high concentrations in the interstitium. Then, it meets hydrogen and chloride in the cortical collecting duct where hydrochloric acid is transformed into ammonium chloride which is a weak acid. This is excreted from the body.
Requirements for Distal H+ Secretion
- Proton pump in the alpha intercalated cell (allowing NH3 to be turned into NH4+)
- Negative luminal charge (created by the principal cell)
- Supply of NH3

Type 1 vs Type 4 RTA
Type 1 RTA - a problem with the alpha intercalated cell. Potassium will be low and the TTKG will be normal.
Type 4 RTA - a problem with the principal cell. Potassium will be high
Type 2 RTA
Is HCO3 wasting
High ammonium in the urine must be a failure to reabsorb bicarbonate. Ammonium should be high in the presence of acidosis.
You can look at the urine net charge to determine the amount of ammonium in the urine. High amounts of chloride go along with high amounts of ammonium. With a negative urine net charge of -20 to -50, this indicates that NH4+ production is okay. With a positive urine net charge, this indicates that NH4+ production is impaired.
Ammonium Levels in Urine
Ammonium cannot be measured directly, instead we need to look at the net charge of the urine. Chloride ions go along with ammonium ions, which we can measure.
If the urine has a net negative charge of -20 to -50, this indicates that ammonium production is okay.
If the urine has a net positive charge, this indicates that ammonium production is impaired and the alpha calated cells or the principal cells could be to blame. We can look at the potassium levels to determine which one (low in alpha calated or normal in principal cell)


