Membrane Potentials Flashcards
(39 cards)
What is the relationship of K+, Na+, and Cl- concentrations inside and outside the cell?
What is standard osmolarity?
- K+ concentration is generally high intracellularly and lower extracellularly, while Na+ and Cl- are high extracellularly and lower intracellularly
- standard osmolarity: 285 mOsM
What are the differences between voltage-gated channels vs. ligand-gated channels?
- voltage gated channels have gates controlled by change in membrane potential (e.g. sodium and potassium channels)
- ligand-gated channels have gates controlled by binding of ligand, such as a NT (e.g. acetylcholine receptors)
- potential difference generated across membrane when ion diffuses down conc gradient
- magnitude of diffusion depends on size of conc gradient
- conc gradient is driving force
- usually measured in millivolts (mV)
diffusion potential
What determines equilibrium potential within a cell?
- chemical forces (conc gradient) and electrical forces (electrical potential)
- can be mathematically calculated by the Nernst equation
What is the simplified Nernst equation? (yes, you do need to know this and be able to use it)
(when RT/F at 37 degrees = 26.8 mV and ln(x) = 2.3log(x))

What are the approximate conc of the following ions in ECF and ICF?
a) Na+
b) K+
c) Ca2+
d) Cl-
a) Na+: ECF (140) and ICF (14)
b) K+: ECF (4) and ICF (120)
c) Ca2+: ECF (2.5) and ICF (0.0001)
d) Cl-: ECF (105) and ICF (10)
What are the typical values for equilibrium potential for these ions in skeletal muscle?
a) Na+
b) K+
c) Ca2+
d) Cl-
a) Na+: +65 mV
b) K+: -95 mV
c) Ca2+: +120 mV
d) Cl-: -90 mV
What is the difference in diffusion equilibrium for charged vs uncharged substances?
uncharged substances (e.g. sucrose_ are only influenced by conc gradient as the driving force, electrical force does not influence equilibrium stated in uncharged substances
What must driving force for net diffusion of ions account for?
What is the equation for net driving force?
- must account for conc gradient and electrical potential across membrane
- net driving force (mV) = Em- Ex
(Em = membrane potential (mV) and Ex = equilibrium potential for given ion (mV)
What will occur within the cell if:
- driving force is negative (Em is more negative that Ex):
- driving force is positive (Em is more positive that Ex):
- driving force is zero (Em and Ex are equal):
- driving force is negative (Em is more negative that Ex): cation will enter the cell, anion will leave the cell
- driving force is positive (Em is more positive that Ex): cation will leave the cell, anion will enter the cell
- driving force is zero (Em and Ex are equal): no net movement of ions
What is the equation for ionic current? (current generated by movement of charged ions across membrane)
ionic current = Gx (driving force of given ion)
- this equation is a rearrangement of Ohm’s law equation (V=IR) where V is voltage (same as E) and R is resistance
- since G is the reciprocal of resistance, Ohm’s law equation can be arranged to estimate ionic current: I = G x V
LOL I LOVE HOW I THOUGHT I WOULD BE DONE W/ PHYSICS IN MED SCHOOL, LOLOLOL GUESS NOT
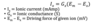
What are the two factors that affect ionic current across cell membrane?
- difference between equilibrium potential for given ion and actual membrane potential (i.e. driving force = Em - Ex): larger the difference between Em and Ex, the larger the imbalance between electrical and conc gradients, thus a larger net movement of given ion
- permeability of membrane to given ion: if perm high, ionic current at particular value of driving force will be higher than if permeability was low, permeability is closely related to conductance (an index of ability of an ion to carry current across a membrane, for a given driven force the greater the conductance the greater the current flow)
What two factors determine the membrane potential?
- ion concentrations: conc gradients (e.g. sodium-potasium ATPase)
- relative ion permeabilities: determine relative importance of particular ion in governing where Em lies, ions w/ highest permeabilities/conductances at rest will make greatest contribution to resting membrane potential (if cell membrane is highly permeable to ion, that ion can respond readily to deviations away from its equilibrium potential and Em will tend to be near that equilbrium potential)
- equation that gives a quantitative relation between Em on one hand and ion conc and permeabilities on other hand
- interpretation: combination of outwardly directed K+ gradient (product of sod-pot ATPase activity) and high permeability of membrane to K+ makes the ICF electrically negative w/ respect to ECF; however, the finite permeability of membrane to Na+ and Cl- prevents the membrane potential from reaching the Nernst potential for K+
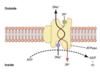
Goldman-Hodgkin-Katz equation

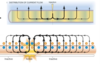
What is the role of the sodium-potassium ATPase pump?
- electrogenic contribution: 3 Na+ ions pumped out of the cell for every 2 K+ ions pumped into the cell
- maintains conc gradients of Na+ and K+
- alternate equation to the Goldman equation
- considers the equilibrium potential for each ion by its relative conductance
- ions w/ highest conductance drive membrane potential toward their equilibrium, ions w/ low conductance have little influence on membrane potential
Chord conductance equation
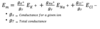
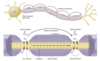
- this potential is generated by a transient dramatic increase in sodium permeability (and conductance) due to the activation of voltage-gated Na+ channels
- this potential is the basic signal of the nervous system
action potential

What are the characteristics of an action potential?
- triggered by depolarization
- threshold level of depolarization must be reached to trigger AP
- all or nothing events
- propagation of AP’s from one site to the next is nondecremental (magnitude is preserved)
- at hte peak of the AP, membrane potential reverses sign becoming inside positive
- after a neuron/cell fire an AP, there is a brief period in which it is impossible to trigger another AP (i.e. absolute refractory period)

Describe the following parts and phases of the action potential:
- resting membrane potential:
- threshold potential:
- depolarizing phase:
- peak:
- overshoot:
- repolarization phase:
- undershoot:
- hyperpolarizing phase:
- resting membrane potential: steady state; cell interior negative (~ -70 mV)
- threshold potential: membrane potential at which the depolarization level is enough to trigger an AP
- depolarizing phase: membrane potential becomes less negative than the resting membrane potential (Na+ influx dominates, causes inward current, becomes self-sustained giving rise to upstroke)
- peak: maximum amplitude (depolarization) of the AP
- overshoot: membrane potential transiently overshoots zero, and the inside of the cell becomes positive w/ respect to the outside for a brief period of time
- repolarization phase: upstroke is terminated, and the membrane repolarizes to the resting level
- undershoot: (hyperpolarizing afterpotential) when the AP repolarizes toward the normal resting membrane potential, it transiently becomes more negative than normal
- hyperpolarizing phase: the membrane potential becomes more negative than the resting membrane potential

What is the relation of ion concentration within the cell and action potential?
- Na+ increases during the action potential, it’s concentration generally follows the same pattern as the action potential does on an mV x time graph, while K+ begins increasing once the AP has reached its peak and K+ is highest when the AP is going into undershoot (declining)
- conduction state of sodium channel depends on membrane potential (since it is a voltage-gated sodium channel)
- when membrane is depolarized, permeability of Na+ increases, allowing Na+ ions to carry positive charge into the cell, this depolarizes cell further, causing greater increase in permeability of Na+ and more depolarization
- this process is explosive and tends to continue until all sodium channels are open and membrane potential has been driven up near equilibrium potential of Na+

What causes the Em (membrane potential) to return to rest after the action potential?
- depolarization-induced increase in permeability of Na+ (pNa) is transient
- there is a delayed, voltage-dependent increase in permeability of K+ (pK)

How do ions (Na+ in particular) control repolarization after an action potential?
- the voltage-dependent sodium channel is controlled by activation/inactivation (acts as if flow of Na+ is controlled by two independent gates)
- activation gate: closed when membrane potential is equal to or more negative than the usual resting membrane potential (prevents Na+ from entering the cell at resting potential)
- inactivation gate: open at resting membrane potential
- both gates: respond to depolarization but w/ different speeds and in opposite directions (act opens rapidly in response to depolarization, inact closes sowly in response to depolarization)
- immediately after depolarization, both gates are open allowing Na+ to enter the cell
- ~1-2 ms after act gate is still open, but inact gate has responded by closing
- as a result: permeability of Na+ first increases in response to depolarization, then declines again even if depolarization were maintained in some way
- delayed decline in Na+ permeability upon depolarization is called sodium channel inactivation

How do the voltage-dependent potassium channels respond during an AP?
- channels are closed at resting membrane potential
- act gates slowly open upon depolarization (slow activation)
- permeability of K+ increases w/ a delay following depolarization, K+ current is outward
Describe the following refractory periods in relation to the AP:
- absolute refractory period:
- relative refractory period:
- absolute refractory period: when inactivation gates of voltage-gated sodium channels are closed, no amnt of depolarization can cause the cell to fire an AP, prevents influx of Na+ necessary to trigger the AP, no AP can occur
- relative refractory period: overlaps w/ hyperpolarization phase (undershoot), during which conductance to K+ is higher than normal, AP can be evoked but only if a greater than usual depolarizing current is applied