Jackson - Colon Polyps to Cancer Flashcards
What are the 7 types of colon polyps?
-
Adenomatous (70%): pre-malignant (how most colon cancers start)
1. Tubular
2. Villous
3. Tubulovillous
4. Sessile serrated -
Non-adenomatous (30%):
1. Hyperplastic
2. Hamartomatous
3. Juvenile polyps

What are these?

- Hyperplastic polyps: most common non-neoplastic polyp
- Histology with saw tooth (star-shaped) pattern (some histo overlap with sessile serrated adenomas)
- Usually diminutive
- Location usually in rectum and sigmoid
- No malignant potential in small distal hyperplastic polyps

What do you see here? Describe the related syndrome.

- Peutz-Jeghers Syn.: multiple GI hamartomatous polyps (sm. intestine) & mucocutaneous lesions (like freckles on buccal mucosa; may go away by age 20)
- Auto dom (present around 11) -> germline hetero LOF mutations in the gene STK11
- Assoc w/markedly INC risk of several malignancies; lifetime risk approx 40%
- Regular surveillance recommended starting at birth:
1. Sex cord tumors of the testes
2. Late childhood gastric, sm. intestinal cancer
3. 2nd-3rd decades of life for colon, pancreatic, breast, lung, ovarian, and uterine cancers - Occasionally sporadic, or as components of various genetically determined/acquired syndromes
What are juvenile polyps? Presentation? Morphogenesis?

- Focal malformations of epi and lamina propria
- Sporadic (usually solitary) or syndromic in kids <5
- Commonly in rectum; present with rectal bleeding
1. Intussusception, intestinal obstruction, polyp prolapse (through anal sphincter) may occur - Pts w/auto dom syndrome can have from 3-100 hamartomatous polyps, and a minority can undergo malignant transformation
- Morphogenesis is incompletely understood, but proposed that mucosal hyperplasia is initiating event
1. Dysplasia is rare in sporadic juvenile polyps
2. 30-50% of pts w/juvenile polyposis syndrome develop colonic adenocarcinoma by age 45
3. Most common mut, SMAD4
What is this? Risk factors? Types? Histo?
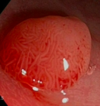
- Adenomatous polyp: prevalence 25-30% at age 50
- RISK FACTORS: age, abdominal obesity, male sex, and AA race
- Can be sessile (front image), pedunculated, flat, or depressed
- HISTO: tubular (80%), villous (5-15%), or mixed (tubulovillous adenoma)
What is the most common type of neoplastic polyp?
- Adenomatous
- Image of pedunculated attached here

What are these?

Villous adenomas
What factors are most important in the prognosis of colon polyps?
- SIZE + villous component
- Usually takes about 10 years for malignant transformation

What do you see here?
- Sessile serrated adenoma: some histo features of hyperplastic polyps, but have malignant potential
- More prevalent in proximal colon
- Typically flat lesions
- May account for missed lesions on colonoscopy
- Have MSI-H or BRAF mutations
- May have mucous cap sitting on top of them

What is going on here?
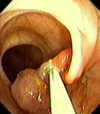
- Polypectomy: removal of a stalked polyp with colonoscopy using cautery and a snare
- Sent to pathology next to check margins
What is the epi of colorectal cancer?
- Most common GI malignancy; mortality 2nd only to lung cancer
- Higher incidence in developed countries: thought to be 2o to high fat, low fiber diet
-
Calcium and folate in diet may be protective
1. Folate may have anti-cancer benefit early in adenoma sequence - Most colorectal tumors are adenocarcinomas
- Incidence of CRC has declined 30% in last decade in patients >50-y/o
1. 5% of Americans will develop CRC and 40% of these will die of the disease - NOTE: cancer of sm. intestine very rare

What are the risk factors for colorectal cancer?
- Age: >50
- Colitis: may not develop colon polyps

What is the most common risk factor for colon cancer?
- Age
- Male = female
- NOTE: AA’s may have earlier age of onset
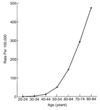
How does family hx affect colon cancer risk?
- Very young relatives, or more than one relative, start thinking about inherited syndromes

What is the influence of tumor location on colon cancer presentation?
- LEFT: obstructive symptoms (smaller lumen), changes in bowel movements, overt bleeding
- RIGHT: iron deficiency anemia or occult blood in stool
- NOTE: when an adult presents with iron deficiency anemia, think about colon or gastric cancer

What is going on here?

- Barium enema: apple-core lesion surrounding lumen of descending colon
- Can be used to diagnose colorectal cancer: will show mass or constricting lesion
What are 2 techniques for diagnosis of colorectal cancer?
- Barium enema: will show mass or constricting lesion
- Colonoscopy: locate & biopsy lesions, and remove polyps

What are some of the txs for colorectal cancer?
- Endoscopic polypectomy can be curative if cancer is localized to head of polyp
- Pre-op CT to look for metastatic disease (mets usually to liver, lungs)
- Surgery: mainstay of treatment -> involves removal of tumor and adjacent lymphatics
- Chemo: adjuvant tx in pts with (+) nodes -> DEC recurrences and improves survival (see attached)

What are the 2 goals and 4 barriers of colorectal cancer screening?
- GOALS:
1. DEC mortality by detecting lesions earlier
2. Prevention by removing adenomatous polyps - BARRIERS:
1. Limited access to medical care/colonoscopy
2. Pt preference: bowel prep, time off from work for colonoscopy
3. Risk and expense of screening tests: best test for individual patient is test that gets done - NOTE: only 65% of patients currently get screening between age of 50 and 75
What are the colorectal cancer screening recommendations based on risk category?
-
Average risk: asymptomatic, age >50
1. US Task Force recommended to stop screening at age 75 -
High risk: asymptomatic + 1 of the below; always require a colonoscopy
1. Personal history of adenomas or cancer
2. Family history of adenomas or cancer
3. Hereditary cancer (FAP, HNPCC)
4. IBD colitis - If any of these screening tests (+), recommended pt have colonoscopy (last 3 are stool sample tests)
- NOTE: if you have symptoms, no longer screening, but rather diagnostic colonoscopy
What is the FOBT?
- Fecal occult blood test (FOBT): stool-based colo-rectal screening test
- (+) test = 20% chance of having lg polyp or cancer
-
80% false positive rate: affected by meds, diet
1. Can also have false negatives b/c pt may not always be bleeding - Requires 3 stool samples
- Low sensitivity for detecting CRC
- NOT__E: FIT is a better test

What is FIT?
- Fecal immunochemistry test: stool-based colo-rectal cancer screening test
- Responds to only human hemoglobin: no dietary restrictions
- Does NOT detect UGI bleeding
- Requires 1 or 2 stool samples, and may detect as little as 0.3gmHb/gm stool
- More expensive than Guiac (FOBT), but also more sensitive and specific
- Can use for low-risk, asymptomatic patients
What is virtual colonoscopy?
- Helical CT reconstructed into 3D images
- Requires bowel prep: most pts dislike this
- Exposure to radiation
- Not widely available, and studies on sensitivity and specificity have been variable
- Role in screening unclear: not paid for by most insurance
1. Positive test requires colonoscopy
Is sporadic or familial colorectal cancer more common? 1o difference?
- Majority of cases SPORADIC (see attached image)
- Cumulative incidence much higher at younger ages in FAP, then HNPCC -> genetic syndromes, as compared with the general public

What is the genetic etiology of FAP?
- Auto dom: one allele of APC gene inherited in a mutated form (germ line mutation)
- Mutation present in every cell of the colon
- Polyp growth begins when 2nd allele is somatically mutated, causing loss of gene function
1. Normally, during teen years

What is FAP?
- Mucosal surface of colon a “carpet” of small, adenomatous polyps (see attached image)
- Typically, total colectomy before age 20 (post-pubertal)
- Polyps look like tubular adenomas histologically
1. Don’t look any different than sporadic, and no higher risk of change, but so many that risk of colon cancer is high - Adenocarcinoma in 100% of these pts if untreated, often before age 30, and always by age 50
- NOTE: adenomas may devo elsewhere in GI tract, esp. adjacent to ampulla of Vater (union of pancreatic and common bile duct) and in the stomach

What are the two syndromic variants of FAP?
- Gardner’s: multiple colon polyps, osteomas, thyroid cancer, desmoid fibromas, epidermal inclusion cysts
- Turcot’s (autosomal recessive): polyps with glioblastoma or medulloblastoma
What is HNPCC?
- Hereditary non-polyposis colon carcinoma/Lynch syn: auto dom -> faulty DNA mismatch repair gene
- Most HNPCC tumors have microsatellite instability (variations in dinucleotide repeat sequences)
1. Compared to APC, in HNPCC, there are fewer polyps and an older age for devo of carcinoma - 2-4% of all colorectal cancers, making it the most common syndromic form of colon cancer
- Tend to occur at younger ages than sporadic colon cancers, and are often located in the right (proximal) colon

What are the Bethesda Criteria for the dx of HNPCC?
- CRC diagnosed in individual < 50
- Presence of other HNPCC-assoc. tumors (small bowel, endometrial, ovarian, gastric, hepatobiliary, transitional cell carcinoma of renal pelvis or ureter), regardless of age
- CRC w/MSI-H histology, in patient <60
- CRC in >1 1st-degree relative w/HNPCC-related tumor, w/1 cancer diagnosed <50
- CRC dx’d in 2 or more 1st or 2nd degree relatives with HNPCC-related tumors, regardless of age
- NOTE: this is a clinical dx that can be confirmed by histology -> currently most GI pathology leaders are screening every colorectal adenocarcinoma for MSI
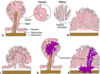
Briefly describe the histology of the benign CRC polyps (image too).
- A: normal
- B: hyperplastic, with sawtooth (serrated, star-shaped) lumens
- C: hamartoma = mass of mature, but disorganized tissue indigenous to site (feature of Peutz-Jeghers syndrome)
- D: juvenile -> called “retention polyps” in adults

What do you see here?

- Hyperplastic polyp: BENIGN, loose, and soft
- Well-formed, elongated glands and crypts with sawtooth (star-shaped) appearance due to DEC epithelial cell turnover, delayed shedding of surface cells, and piling up of cells
- Mixture of goblet cells (with abundant mucin) and absorptive cells
- Bland cytology with eosinophilic cytoplasm
What is this? Histo criteria?
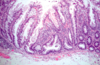
- Sessile serrated adenoma: histo overlap with hyperplastic polyps (differential diagnosis)
- Be on high alert if in the right colon: low threshold for calling these, esp. on the right side
- Malignant potential, but lack typical cytologic features of dysplasia
- Histologic criteria (strict, if on left side) for these lesions include:
1. Serrated architecture throughout full length of the glands, including the crypt base
2. Crypt dilation
3. Lateral growth (boot shape)

What histo is characteristic of Peutz-Jeghers Syndrome polyps?
- Large, pedunculated polyps with characteristic arborizing network of connective tissue -> smooth muscle, lamina propria and glands lined by normal-appearing intestinal epithelium
- Complex glandular architecture, and presence of smooth muscle are features that distinguish Peutz-Jeghers polyps from juvenile polyps
- Stalk with smooth muscle bands and architectural complexity
- REMEMBER: P-J is auto dom, and characterized by devo of benign hamartomatous polyps in GI tract and hyperpigmented macules on lips and oral mucosa

What kind of polyp is this?

- Peutz-Jegher’s syn polyp: large, pedunculated polyps characteristic arborizing network of CT -> smooth muscle, lamina propria and glands lined by normal-appearing intestinal epithelium
- Complex glandular architecture, and presence of smooth muscle are features that distinguish Peutz-Jeghers polyps from juvenile polyps
- Stalk with smooth muscle bands and architectural complexity
- REMEMBER: P-J is auto dom, and characterized by devo of benign hamartomatous polyps in GI tract and hyperpigmented macules on lips and oral mucosa
What are these?

- Juvenile polyps: pedunculated, smooth-surfaced, reddish lesions w/characteristic cystic spaces (image)
- Most are <3 cm in diameter
- Microscopic examination shows: surface erosion
1. Dilated glands filled with mucin and inflam debris
2. Lamina propria expanded by mixed inflam infiltrates - Do NOT have arborizing appearance or smooth mm
- Granulation tissue on top many times b/c they have been beaten up

What kind of polyp is this? Arrow?

- Juvenile polyp: inspissated mucous, neutrophils, and inflammatory debris can accumulate within dilated crypts
How do adenomas progress to carcinoma?
- An adenoma is already LOW-GRADE DYSPLASIA
- No lymphatics in the pedunculation, so if there is early carcinoma confined to the polyp, patient should be fine after removal (D)
What is this?

- Tubular adenoma: tend to be small, pedunculated polyps composed of rounded, or tubular, glands
What do you see here?
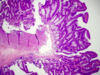
- Tubulovillous adenoma: mixture of tubular and villous elements
- 1-20% villous component = higher rate of of p53 and KRAS mutations and MGMT loss
What is this?

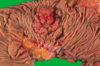
- Villous adenoma: often larger, and sessile, and covered by slender villi (long, finger-like projections)
- Cauliflower-like structure (see attached) due to elongated glandular structures covered by dysplastic epithelium
What are the 3 histo categories of CRC adenomas? Significance? Most important factor?
- Tubular, tubulovillous, and villous -> categories have little clinical significance in isolation
- Although villous adenomas contain foci of invasion more frequently than tubular adenomas, villous architecture alone does not INC cancer risk when polyp size is considered
- SIZE is always biggest consideration (villous tend to be a little larger; also may have higher rate of mutations -> 1-20% villous component = higher rate of of p53 and KRAS mutations and MGMT loss)

What grade of dysplasia (if any) do you see here? Why?

- Tubular adenoma w/low-grade dysplasia
- Hallmarks of epithelial dysplasia:
1. Nuclear hyperchromasia
2. Elongation
3. Stratification
What grade of dysplasia do you see here? Why? Most important prognostic indicator?
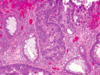
-
High-grade dysplasia: cells no longer organized, hardly any lumen due to lost polarization, pleomorphic/enlarged nuclei
1. At least high-grade bc can’t rule out invasion (could just be where they took biopsy) - Hallmarks of epithelial dysplasia:
1. Nuclear hyperchromasia
2. Elongation
3. Stratification - Polyp size is the most important characteristic that correlates with risk of malignancy
What is going on here?

- Adenocarcinoma: large, crowded cells, nuclei, and nucleoli + decreased cytoplasm
- Abnormal, irregular, and complex gland structures invading muscular wall (see attached image)
- Apoptotic debris and necrosis b/c cells dividing so rapidly they are dying; clumpy chromatin
- NOTE: functional lymphatic channels absent in colonic mucosa

What is this?

- Adenocarcinoma: pools of mucin with strips of carcinoma
What do you see here?

- Mucinous + signet ring features in colonic adenocarcinoma
What is this?

- Signet ring morphology of colon adenocarcinoma
What is going on here?

- Dirty necrosis of metastatic CR adenocarcinoma
- Lymph node with architecture obliterated due to metastatic adenocarcinoma
1. Apoptotic debris, neutrophils hanging out with tumor cells in the attached image - Adenocarcinoma of the colon tends to be a little bit taller cells
- NOTE: the two most important prognostic factors for CRC are depth of invasion and the presence of lymph node metastases

What are the 2 most important prognostic factors for CR adenocarcinoma?
- Poorly differentiated and mucinous histo are assoc with poor prognosis
- But, the two most important prognostic factors are:
1. DEPTH OF INVASION
2. PRESENCE OF LYMPH NODE METS
Where does CRC met to? Worst prognosis?
- Liver, lung, peritoneum
- With the exception of brain metastasis, liver mets had the worst prognosis (median survival = 9 mos)
How is CRC staged? Effect on prognosis?
- STAGING: also a system for nodes and mets (distant invasion comes with a much worse prognosis)
1. T1 (top): invading submucosa
2. T2 (middle): invading into, but not through muscularis propria
3. T3 (bottom): invading through muscularis propria (MP); yellow arrow points to malignant glands in serosa

What stage of adenocarcinoma is this?
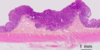
- T1: colon carcinoma invading the submucosa
- NOTE: T0 would be CIS
What stage of adenocarcinoma is this?

- T2: invading into, but NOT through the muscularis propria
What stage of adenocarcinoma is this?

- T3: colon cancer invading through the muscularis propria (MP)
- Yellow arrow points to malignant glands in serosa
- NOTE: T4 would be invasion of other organs or structures, and/or perforation of the visceral peritoneum
What are the 3 pathways to colorectal cancer, and their associated mutations?
- CLASSIC: 80% of sporadic colon cancers
1. APC -> KRAS -> p53
2. Mutant KRAS renders tumors resistant to EGFR-targeted therapies - MICROSATELLITE INSTABILITY (MI): Lynch syndrome -> mismatch repair
- SESSILE SERRATED: CpG island hypermethylation
1. BRAF mut almost exclusively seen in sporadic MSI tumors presumed to develop through the serrated tumorigenic pathway, but has never been reported in Lynch syndrome

Describe the progression of the classic CRC pathway.
- 80% of sporadic colon cancers
- APC -> KRAS -> p53
- Mutant KRAS renders tumors resistant to EGFR-targeted therapies

Describe the (genetic and histo) progression of the Lynch CRC pathway.
- Patients with HNPCC inherit one mutant gene and one normal allele
- When 2nd copy is lost via mutation or epigenetic silencing, defects in mismatch repair lead to the accumulation of mutations at rates up to 1000 times higher than normal, mostly in regions containing short repeated sequences called microsatellites
- Majority of HNPCC (90%) and subset of non-HNPCC patients have germline mutations in DNA mismatch repair (MMR) genes

What are 2 screening techniques for HNPCC?
- Screening by IHC (see attached image): most places start with this, then do PCR if they have to
- Screening by MSI (PCR)
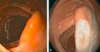
Diagnosis? Descending colon.

Hyperplastic polyp
Diagnosis?

Tubular adenoma
Diagnosis?

Villous adenoma
Diagnosis?

Sessile serrated adenoma
Stage?

T3
This syndrome is a variant of FAP. The patient has desmoid tumors and osteomas of the forehead. What is the syndrome and gene?
Gardner’s syndrome: APC
The molecular defect in Hereditary nonpolyposis colorectal cancer/Lynch syndrome is…?
DNA mismatch repair
What is the key mutation in FAP? Consequences?
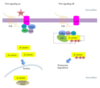
- Loss of APC, which normally binds to, and promotes degradation of B-catenin
- Steps with loss of APC function:
1. β-catenin accumulates and translocates to the nucleus,
2. Where it forms a complex w/DNA-binding factor TCF and activates the transcription of genes, including MYC and cyclin D1, that
3. Promote proliferation