Glomerulonephritis Flashcards
pathogenesis
immunologically mediated disorder
- humoral - Ab driven
- cell mediated (T cells)
- involvement of inflammatory cells, mediators and complements
define
immune mediated disorder of the kidneys affecting the glomeruli, with secondary tubulo-interstitial damage
name 3 consequences of inflammation of the glomerulus
- damage to the glomerulus restricts blood flow, leading to compensatory increase in systemic BP
- damage to the filtration mechanism allows protein and blood to enter the urine
- loss of the usual filtration capacity leads to AKI
what does damage to endothelial cells lead to
- vasculitis - most aggressive form of inflammation in the kidney, can lead to AKO
- haematuria

what does damage to mesangial cells lead to
- proliferative lesion, release of angiotensin II which causes vasoconstriction
- chemokines are released which attract inflammatory cells
- haematuria

what does damage to podocytes cause
- non-proliferative lesion - podocytes atrophy, lose filtration barrier
- protein in urine

broad presentation of GN
- specific syndrome eg nephrotic or nephritic
- blood pressure
- urine dipstick: protein/blood
- renal function impairement
compare the presentation of nephritic syndrome to nephrotic
- nephrotic syndrome: Defined as proteinuria >3g/d, hypoalbuminuria (<25g/L) and oedema with severe hyperlipidaemia (and cholesterol). This is indicative of a non-proliferative process affecting podocytes

nephritic syndrome
syndrome comprising signs of nephritis, often occurs in GB
aetiology
- the majority are primary with no underlying drive to disease
- 2y - infections, drugs, autoimmunity, malignancy, systemic disease
define focal and diffuse
- focal <50% glomeruli affected
- diffuse >50%
define global and segmental
all/part of glomerulus affected
focal lesions are often segmental
crescenteric
presence of crescents:
- Glomerular disease leads to gaps or holes in the GBM resulting in epithelial cell proliferation with mononuclear infiltration in Bowman’s space. Ultimately, fibrocellular crescent formation
- these are associated with RPGN
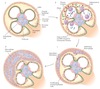
how does IgA nephropathy present
- macro or microscopic haematuria ± nephritic syndrome
what is the most common GN in the developed world
IgA nephropathy
pathology of IgA nephropathy
- there is raised abnormal IgA (possibly due to infection of mucosal membrane)
- target by the immune system and forms immune complexes with IgG
- these are deposited in mesangial cells
- accumulate and cause local immune ativation - proinflammatory cytokine release and macrophages come - and injury
- RBS pass through into urine
when does IgA nephropathy typically develop
during infection of mucosal membrane eg resp/GI tract
how does IgA nephropathy progress
- over time due to glomerular inflammation and injury, patients progress to renal failure
- 25% progress to ESRF in 10-30 years
describe a typical patient with IgA nephropathy
- Typical patient: young man with episodic macroscopic haematuria after respiratory/G.I. infection, recovery is rapid between attacks
what is IgA Nephropathy associated with systemically
Henoch Schonlein purpura
biopsy of IgA Nephropathy
mesangial proliferation and expansion
IF and EM of IgA Nephropathy
immune deposits (IgA and C3) are seen in mesangium
H&E stain of IgA Nephropathy
mesangial cell proliferation and expansion
treatment of IgA Nephropathy
the aim is to prevent further damage and avoid ESRF
- control BP: ACEi/ARB, fish oil
- prevent immune complex formation: corticosteroids












