First Semester Exam Flashcards
(12 cards)
Why do we balance equations?
So that they will follow the law of mass conservation.
When do we use paraenthasies in equations?
When there is more than one polyatomic ion in a formula.
What are three ways you can tell chemical reaction has happend?
When properties of the chemical change. (ex. Formation of a gas, a change in color, or the release of heat or light.)
What is a substance that changes a reaction rate without being permanently changed by the reaction?
Catalyst
What symbol is someines used to indicate a solid product?

What symbol is used to indicate a liquid product or reactant?
(l)
Identify the type of reactant.
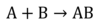
Synthesis Reaction
Identify type of reactant.

Decomposition Reactant
Identify type of reactant.

Double Replacement Reactant
Identify type of reaction.

Single replacement reaction
What symbol indicates that heat must be supplied to reactant before a reaction?



