43: Pediatric Surgery Flashcards
What is the most common solid abdominal malignancy in children?
Neuroblastoma
[Usually presents as an asymptomatic mass but can present with secretory diarrhea, raccoon eyes (orbital metastases), HTN, and opsomyoclonus syndrome (unsteady gate).]
[UpToDate: The term neuroblastoma is commonly used to refer to a spectrum of neuroblastic tumors (including neuroblastomas, ganglioneuroblastomas, and ganglioneuromas) that arise from primitive sympathetic ganglion cells and, like paragangliomas and pheochromocytomas, have the capacity to synthesize and secrete catecholamines.
Neuroblastomas, which account for 97% of all neuroblastic tumors, are heterogeneous, varying in terms of location, histopathologic appearance, and biologic characteristics. They are most remarkable for their broad spectrum of clinical behavior, which can range from spontaneous regression, to maturation to a benign ganglioneuroma, or aggressive disease with metastatic dissemination leading to death.
Clinical diversity correlates closely with numerous clinical and biological factors (including patient age, tumor stage and histology, and genetic and chromosomal abnormalities). For example, most infants with disseminated disease have a favorable outcome following treatment with chemotherapy and surgery, although the majority of children older than one year of age with advanced-stage disease die from progressive disease despite intensive multimodality therapy. This clinical complexity likely derives from the developmental origins of neuroblastoma, which arises due to developmental arrest of maturing components of the embryonic neural crest.
Neuroblastoma has been associated with central hypoventilation, Hirschsprung disease, and neurofibromatosis type 1 (neurocristopathy syndrome), and as a familial disorder associated with mutations in the ALK gene.]

What does an increase in alkaline phosphatase indicate in children?
Bone growth
What is the appropriate treatment of a thyroglossal duct cyst (TGDC)?
Excision of the cyst, tract, and hyoid bone (at least the central portion)
[UpToDate: Infection is the most common complication of TGDC. An infected TGDC typically presents as a tender mass, with or without fever, and may have a draining sinus; it should be managed initially with antibiotics, followed by definitive surgery once the infection has resolved. The standard surgical treatment is resection of the cyst and the midportion of the hyoid bone in continuity and resection of a core of tissue from the hyoid upwards towards the foramen cecum, an operation known as the Sistrunk procedure.]

The midgut includes which structures?
- Duodenum distal to ampulla
- Small bowel
- Large bowel up to distal 1/3 of transverse colon

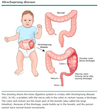
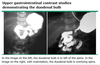
What percent of congenital diaphragmatic hernias occur on the left side?
80%
[80% have associated anomalies (cardiac and neural tube defects mostly)]
[UpToDate: In most cases of CDH, herniation occurs on the left. Right-sided diaphragmatic hernias occur in approximately 15% of cases and bilateral herniation in 2%. Although there is no difference in mortality between left- and right-sided lesions, there may be a higher incidence of pulmonary complications associated with right- versus left-sided CDH.]

How many degrees and in what direction does the midgut rotate during embryonic development?
270 degrees counterclockwise

What is the appropriate treatment of pyloric stenosis?
Pyloromyotomy
[RUQ incision; proximal extent should be the circular muscles of stomach.]
[UpToDate: The classical operation for IHPS is Ramstedt pyloromyotomy, which involves a longitudinal incision of the hypertrophic pylorus with blunt dissection to the level of the submucosa; it relieves the constriction and allows normal passage of stomach contents into the duodenum. Laparoscopic pyloromyotomy is a minimally invasive version of the Ramstedt procedure that has been associated with a lower incidence of postoperative emesis and a shorter hospital stay, but occasionally results in incomplete pyloromyotomy. A transumbilical approach also may be used but has longer operating time.
Open and laparoscopic pyloromyotomy were compared in a prospective trial in 200 infants with ultrasonographically confirmed IHPS, who were randomly assigned to open or laparoscopic pyloromyotomy. There were no differences between groups in operating time, time to full feeding, or length of stay. However, infants in the laparoscopic group had fewer episodes of emesis (2.6 vs 1.9) and received fewer doses of analgesia (2.2 vs 1.6) than those in the open group. A similar randomized study also reported more rapid return to enteral feeding and shorter hospital stay among infants treated laparoscopically, although in 3-5% of laparoscopically performed cases the pyloromyotomy was incomplete. The study was performed at six centers with extensive experience in laparoscopic techniques.]

What are the indications for surgery in an infant with necrotizing enterocolitis?
- Free air
- Peritonitis
- Clinical deterioration
[UpToDate: When the diagnosis of necrotizing enterocolitis (NEC) is suspected or confirmed, a pediatric surgeon is consulted to assist the neonatology team in the evaluation and management of the infant, and to decide if and when surgery is needed. The timing of surgical intervention in a critically ill infant requires considerable judgment, as one wishes to preserve as much bowel length as possible, but an unstable patient may not be able to tolerate the surgical procedure.
Infants with NEC require surgical intervention when necrosis extends through the bowel wall and results in perforation. The decision to perform surgery is clear when pneumoperitoneum is recognized on the abdominal radiograph. However, peritonitis, extensive necrosis, or perforation can occur without evidence of free air on the radiograph. As a result, other signs that indicate peritonitis must be considered, including unremitting clinical deterioration, the presence of an abdominal mass, ascites, or intestinal obstruction.]

What are the staging criteria for neuroblastoma?
- Stage 1: Localized, complete excision
- Stage 2: Incomplete excision but does not cross the midline
- Stage 3: Crosses midline +/- regional nodes
- Stage 4: Distant metastases (nodes or solid organ)
- Stage 4-S: Localized tumor with distant metastases
[NSE, LDH, HVA, Diploid tumors, and N-myc amplification (>3 copies) have a worse prognosis.]
[UpToDate:

Which branchial cleft cyst occurs at the angle of the mandible and may connect with the external auditory canal?
1st branchial cleft cyst
[Often associated with the facial nerve.]
[UpToDate: First branchial cleft cysts account for less than 1 percent of branchial cleft anomalies. They typically appear on the face near the auricle. First branchial cleft cysts are further divided into types I and II. Type I first branchial cleft cysts are duplication anomalies of the external auditory canal and are of ectodermal origin. They pass through the parotid gland often in close proximity to the facial nerve. Type II branchial cleft cysts are more common and typically present below the angle of the mandible. They contain both ectoderm and mesoderm and pass through the parotid gland medial or lateral to the facial nerve and end either inferior to the external auditory canal or at the bony cartilaginous junction of the external auditory canal.]

What is the appropriate treatment for gastroschisis?
- Initially place saline-soaked gauzes and resuscitate the patient (can lose alot of fluid from the exposed bowel), TPN, NPO
- Repair when patient is stable. Attempt to place bowel back in abdomen (may need vicryl mesh silo)
- Primary closure at a later date if silo used
[UpToDate: Delivery room — Inflammation and fibrosis from chronic exposure to amniotic fluid result in thickening, matting of the intestines, and decreased bowel motility, and possibly luminal obstruction. Neonatal fluid losses are 2.5 times that of a healthy newborn in the first 24 hours of life. The neonate is at risk for insensible heat and fluid losses from exposure of the eviscerated bowel. In addition, third space fluid deficits from sequestration of intestinal fluid can be significant. The initial approach to management of these newborns includes:
- Wrapping the bowel with sterile saline dressings covered with plastic wrap. This preserves body heat and minimizes insensible fluid loss.
- Inserting an orogastric tube to decompress the stomach.
- Placement of peripheral intravenous access to provide fluids and broad-spectrum antibiotics that cover maternal vaginal flora (eg, ampicillin and gentamicin). The maintenance fluid requirement is increased two- to three-fold because of losses from the exposed bowel.
- Ensuring a patent airway.
- Keeping the neonate in a thermoneutral environment.
Synopsis of surgical management — In the operating room, the bowel is decompressed by aspirating stomach contents and evacuating the large bowel through the rectum. The size of the defect is increased 1 to 2 cm to minimize trauma to the bowel during reduction. The abdominal wall is manually stretched, and the bowel is replaced, taking care to avoid creating intra-abdominal pressure that is too high. Although primary closure is successful in 70% of cases, if it is unsuccessful, a staged closure with a silastic silo can be used, as in omphalocele cases. If primary closure is unlikely to be successful, a preformed silo with a spring-loaded ring can be placed at the bedside to cover the herniated intestine quickly without suturing.
Prolonged postoperative dysmotility is a common problem and interferes with enteral feeding. Studies in animal models suggest that dysmotility is due to delayed maturation of the enteric nervous system, possibly as a result of prolonged exposure to amniotic fluid.]

How is biliary atresia diagnosed?
Liver biopsy (shows periportal fibrosis, bile plugging, eventual cirrhosis)
[Ultrasound and cholangiography can reveal atretic biliary tree]
[UpToDate: The diagnosis of BA is made with a series of imaging and laboratory tests and liver biopsy to exclude other causes of cholestasis. Infants should be evaluated as rapidly as possible because the success of the surgical intervention diminishes progressively with older age at surgery. Because timing is crucial, some infants (eg, those who are eight weeks or older or with a high clinical suspicion of BA) may not require each diagnostic step.
The definitive diagnosis of BA is made by a cholangiogram. This is typically performed intraoperatively; if the diagnosis of BA is confirmed, the surgeon performs a hepatoportoenterostomy (HPE, Kasai procedure).]

What is the most common subtype of bronchopulmonary sequestration?
Intralobular sequestration (also known as intrapulmonary sequestration)
[UpToDate: Congenital abnormalities of the lower respiratory tract are rare, found in about 1 in 10,000 to 35,000 live births. Among these, the most common is congenital pulmonary airway malformation (CPAM), while BPS represents only 0.15 to 6.4 percent. In several reports, even tertiary care referral centers diagnose less than one case per year of BPS.
Intralobar sequestration (ILS) is overall the most common form, comprising approximately 75 to 90 percent of sequestrations, while the remainder are extralobar sequestration (ELS). The difference in prevalence of the disorders may be related to the pathogenic mechanisms, as discussed below. Males and females are equally affected with ILS, while ELS has a male predominance in most, but not all, reports. In a series of ELS cases diagnosed antenatally, the ratio of males to females was three to one. In contrast, bronchopulmonary-foregut malformation (BPFM) has a female predominance.]
What percent of cases of Wilms tumor (nephroblastoma) are bilateral?
10%
[Can be differentiated from neuroblastoma on abdominal CT because it replaces renal parenchyma rather than displacing it.]
[UpToDate: Most patients have solitary Wilms tumor, 5-7% have bilateral kidney involvement, and 10% have multifocal loci within a single kidney. Tumor histology is linked to patient outcome. The classic favorable histology Wilms tumor is comprised of three cell types (blastemal, stromal, and epithelial cells). Anaplasia is associated with poor outcome.]

Which congenital condition is characterized by a failure of cartilage to develop in the bronchus, leading to air trapping with expiration?
Congenital lobar emphysema (Also congenital lobar overinflation and infantile lobar emphysema)
[UpToDate: Progressive lobar hyperinflation is likely the final common pathway that results from a variety of disruptions in bronchopulmonary development. These result from abnormal interactions between embryonic endodermal and mesodermal components of the lung. Disturbances may lead to changes in the number of airways or alveoli or alveolar size. However, a definitive causative agent cannot be identified in approximately 50 percent of cases.
The most frequently identified cause of congenital lobar emphysema (CLE) is obstruction of the developing airway, which occurs in 25 percent of cases. Airway obstruction can be intrinsic or extrinsic, with the former more common. This leads to the creation of a “ball-valve” mechanism in which a greater volume of air enters the affected lobe during inspiration than leaves during expiration, producing air trapping.
Intrinsic obstruction often is caused by defects in the bronchial wall, such as deficiency of bronchial cartilage. This results in airway collapse during expiration. Intraluminal obstruction caused by meconium or mucous plugs, granulomas, or mucosal folds can cause partial obstruction of a lower airway. Extrinsic compression may be caused by vascular anomalies, such as a pulmonary artery sling or anomalous pulmonary venous return, or intrathoracic masses, such as foregut cysts and teratomas. Additionally, bronchial atresia has been identified as a common finding in CLE and other congenital cystic pulmonary malformations.
Males appear to be affected more than females, in a ratio of 3:1. The reason for the male predominance is unknown.
Congenital lobar emphysema (CLE) is characterized by overdistention of one or more lobes of the lung. This leads to compression of the remaining lung tissue and herniation of the affected lobe across the anterior mediastinum into the opposite chest, causing displacement of the mediastinum.]

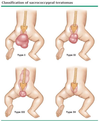
What is the appropriate treatment for a hydrocele?
Surgery (resect hydrocele and ligate processus vaginalis) at 1 year if not resolved or if thought to be communicating (waxing and waning in size)
[UpToDate: The most common treatment is surgical excision of the hydrocele sac. Simple aspiration is generally unsuccessful due to rapid reaccumulation of fluid. On the other hand, percutaneous aspiration of the hydrocele fluid may be successful if combined with instillation of a sclerosing agent into the sac. The potential risks of the latter approach are a low incidence of reactive orchitis/epididymitis and a higher rate of recurrence, which may then make open surgery more difficult because of the development of inflammatory adhesions between the hydrocele sac and the scrotal contents.
Hydroceles discovered in infancy are usually “communicating,” since they are associated with a patent processus vaginalis, which allows flow of peritoneal fluid into the scrotal sac. They usually disappear in the recumbent position and are often associated with herniation of abdominal contents (indirect hernia) through the processus vaginalis. Surgical repair is advised in these cases.]

The foregut includes which structures?
- Lungs
- Esophagus
- Stomach
- Pancreas
- Liver
- Gallbladder
- Bile duct
- Duodenum proximal to ampulla

What is the most common lung tumor in children?
Carcinoid
What should the resuscitation strategy be in an infant with pyloric stenosis that presents with severe dehydration?
Normal saline boluses until making urine, then switch to D5 normal saline with 10 mEq potassium maintenance
[Avoid fluid resuscitation with K-containing fluids in children with severe dehydration as hyperkalemia can quickly develop. Avoid non-salt-containing solutions in infants, as hyponatremia can quickly develop. Infants should always have a maintenance fluid with glucose because of their limited reserves for gluconeogenesis and vulnerability for hypoglycemia.]
[UpToDate: Infants presenting with normal electrolyte values and mild dehydration, as is the case with more than 60% of patients, should receive maintenance intravenous fluids such as 5% dextrose with ¼ normal saline (0.22% NaCl) and 2 mEq KCl per 100 mL. Infants with moderate or severe dehydration require more intensive fluid management with higher NaCl concentrations (½ normal saline [0.45% NaCl] or normal saline [0.9% NaCl]) and higher rates of administration (1.5 to 2 times maintenance), perhaps combined with initial administration of a fluid bolus. In severely dehydrated infants, kidney function should be assessed prior to adding potassium to the intravenous fluids. If alkalosis is present, this should be corrected prior to surgery because it has been associated with an increased risk of post-operative apnea.]
What is the most common overall childhood malignancy?
Leukemia (ALL)
[UpToDate: Acute leukemia, the most common form of cancer in children, comprises approximately 30 percent of all childhood malignancies, with acute lymphoblastic leukemia (ALL) being five times more common than acute myeloid leukemia (AML). Each year in the United States approximately 2500-3500 new cases of ALL are diagnosed in children. ALL incidence is slightly higher in Whites (36 cases/million) and Hispanics (41 cases/million) than in Black Americans (15 cases/million).
Survival rates for ALL have improved dramatically since the 1980s, with a current five-year overall survival rate estimated at greater than 85%. This improvement is in large part because of treatment of large numbers of children with sequential collaborative standardized research protocols. Approximately 75-80% of children with newly diagnosed ALL participate in clinical research trials, the goals of which are to improve clinical outcome and to minimize acute toxicities and late-occurring adverse events.]
What is the appropriate treatment for tracheomalacia?
Aortopexy (aorta sutured to the back of the sternum, opens up trachea)
[Surgical indications include dying spell, failure to wean from ventilator, recurrent infections.]
[UpToDate: The long-term prognosis of this disorder is good in children with no associated problems. Most affected infants improve spontaneously by 6-12 months of age as airway caliber increases and cartilage develops. However, some remain symptomatic or have exercise intolerance as adults.
Intervention may be needed in children with life-threatening episodes of airway obstruction, recurrent infection, respiratory failure, or failure to thrive. Continuous positive airway pressure (noninvasive or invasive via tracheostomy) are the most widely used therapies, although pharmacotherapy also has been suggested. Surgical approaches such as tracheal reconstruction, placement of a tracheal stent, and surgical suspension of the trachea (tracheopexy) also have been reported. Some literature suggests potential roles for airway stents in children, though the use of airway stents typically is reserved for patients whose prognosis is otherwise grim. The development of new 3D-printed personalized external airway stents may make this approach more viable in the future. The relatively low use of stents is due to the frequency of problems related to their use. Expanding metallic stents, in particular, have been associated with a high incidence of complications and are used only in life-threatening situations. Aortopexy, the surgical suspension of the aorta from the sternum, has been reported as an effective treatment for severe tracheomalacia due to a number of different etiologies. In addition to decreasing the compression of the trachea caused by some vascular malformations, aortopexy pulls the anterior tracheal wall toward the sternum, improving airway patency.]

How is Hirschsprung’s disease diagnosed?
Rectal biopsy (absence of ganglion cells in myenteric plexus)
[Occurs due to failure of neural crest cells (ganglion cells) to progress in caudad direction.]
[UpToDate: Hirschsprung disease (HD) is suspected based on clinical features described above, usually supported by contrast enema or anorectal manometry. The diagnosis is established by rectal biopsy.
A suction rectal biopsy can be done at the bedside or in an ambulatory setting without the need for general anesthesia. A biopsy should be taken 2 cm above the level of the dentate line to avoid the 1-2 cm zone of physiologic aganglionosis that is normally present. A second biopsy should be taken proximal to the first one. Adequate tissue is obtained for analysis in the majority of patients. Repeat suction biopsies or full-thickness biopsies under general anesthesia can be performed if the initial biopsy is nondiagnostic (ie, if insufficient tissue is obtained).
The diagnosis of HD is established if ganglion cells are absent in the rectal biopsy, provided that the tissue sample is adequate. Supportive findings include the presence of hypertrophic nerve fibers, elevated acetylcholinesterase activity, which can be seen with special stains, and decreased or absent calretinin-immunoreactive fibers in the lamina propria.
A normal rectal biopsy virtually excludes HD, provided that the biopsy samples are obtained from the correct site and contain at least a small amount of muscularis mucosae. Thus, a rectal suction biopsy is more sensitive and specific than contrast enema and anorectal manometry for the diagnosis of HD for children up to three years of age.]

What is the appropriate treatment for neuroblastoma?
Resection (adrenal gland and kidney taken; 40% cured)
[Initially unresectable tumors may be resectable after doxorubicin-based chemo. Rarely metastasizes, but goes to lung and bone when it does.]
[UpToDate: For children with low-risk disease, surgery is the primary treatment modality when complete resection is feasible, with several exceptions.
- For patients with low-risk tumors that cannot be completely resected or which have life-threatening complications, chemotherapy and/or radiation therapy may be required.
- In the subset of patients with asymptomatic 4S disease, observation may be an option, since there is a high rate of spontaneous regression.
- For infants younger than six months of age with small, localized adrenal masses, we recommend expectant observation with serial ultrasound and urine catecholamines.
For children with intermediate-risk disease, a combined modality approach that includes chemotherapy and surgical resection is standard. The degree of surgical resection required is under investigation. The role of radiation therapy is less clear, except in the context of disease progression despite chemotherapy plus surgery or for complications such as spinal cord compression.
For children with high-risk neuroblastoma, substantial improvements have been seen with aggressive combined modality approaches. These generally include chemotherapy, surgical resection, high dose chemotherapy with stem cell rescue, radiation therapy and biologic/immunologic therapy (eg, dinutuximab). These approaches have improved event free survival, but the majority of patients eventually relapse and die of their disease.
Whenever possible, children with high-risk neuroblastoma should be enrolled in randomized controlled trials in order to validate and improve the long-term efficacy of the current treatment approach.
Children who have been treated for neuroblastoma are at risk for recurrence and for late complications of their treatment. Treating physicians should be aware of these potential issues.]

What is the appropriate treatment for a sacrococcygeal teratoma (SCT)?
Coccygectomy and long-term follow up
[90% benign at birth (almost all have exophytic component). Great potential for malignancy. AFP is a good marker. 2 month mark is a huge transition: Usually benign < 2 months, usually malignant > 2 months.]
[UpToDate: For patients with mature and immature teratomas without malignant elements, we recommend complete surgical resection (Grade 1B). There does not appear to be a role for adjuvant chemotherapy following surgery.
For patients with SCTs that contain malignant elements, we recommend surgical resection, when this can be accomplished without excessive surgical morbidity, followed by adjuvant chemotherapy (Grade 1B). We suggest adjuvant chemotherapy with a platinum-based regimen such as BEP or BEJ, rather than alternative chemotherapeutic regimens (Grade 2B).
- For patients who undergo a complete resection for an SCT with malignant elements, observation, rather than adjuvant chemotherapy, may be an alternative, although there has been limited prospective evaluation of this approach.
For patients with locally advanced or metastatic malignant SCTs, we recommend neoadjuvant chemotherapy prior to resection (Grade 1B).
- For patients who are treated with either adjuvant or neoadjuvant chemotherapy, we suggest a platinum-based regimen, rather than alternative chemotherapeutic regimens (Grade 2C).]