27-09-21 - Structure of Proteins 1 Flashcards
What are the 5 main functions of proteins?
- Provide structure – collagen – the protein of bone, skin and tendons
- Transport molecules – Haemoglobin – the oxygen carrier
- LDL and LDL Receptor – transport cholesterol molecules and co-ordinate their uptake into cells
- Defence – antibodies – defence against infection
- Biological catalysts – enzymes – regulation of all biological systems
- Regulation of genes – Lac repressor – helps control gene expression
How is protein involved in structure?
What is their structure like?
What is their function?
Where are they found
- Collagen fibres are the most abundant protein in mammals (25-35% of total protein)
- Strong flexible protein with fibrous structure
- They are the main component of connective tissues
- Found in skin, tendons, collagen, bone

How is protein involved in transport molecules?
What is the structure of this protein?
What is a prosthetic group?
- Haemoglobin is a protein responsible for selective delivery of O2 to metabolic tissues
- It consists of 4 subunits, with each one containing a haem group that can bind one oxygen molecule
- Haem is an example of a prosthetic group
- A prosthetic group is a non-protein structure that is a vital part of the protein, in this case, haem is iron.

What is another way in which protein is involved in transport molecules? => ALDC
what is the protein made of => appoline + membrane
What do these proteins do?
What happens if these proteins do not work?
- Low-density lipoprotein (LDL) is composed of a phospholipid shell and a single molecule of apolipoprotein B.
- This is used to transport cholesterol between cells via the circulatory system.
- The uptake of LDL particles is mediated the LDL receptor that binds LDL and facilitates internalisation via receptor mediated endocytosis
- A mutation that results in a defective LDL receptor causes familial hypercholesterolemia (too much blood cholesterol)
- Atherosclerosis is a build-up of plaque in the arteries and can be caused by high cholesterol

How are proteins involved in defence? What is their structure like?
- Antibodies are examples of y -shaped glycoproteins
- They consist of two identical heavy chains (red), two identical light chains (blue) and a carbohydrate (usually sugars) covalently linked by disulphide bonds.
- The antigen recognition site is highly specific and tightly binds to the complementary antigen, allowing recognition of proteins by the immune system.

How are proteins involved in being biological catalysts?
example of cleaving enzyme
what happens to enzyme at the end of the reaction
- Proteins make up enzymes, such as lysozymes
- Lysozymes catalyse the cutting of polysaccharide chains
- It bonds to the chains, catalyses the cleavage of a specific covalent bond and released the cleaved products
- The lysozyme remains unchanged at the end of the reaction

How are proteins involved in the regulation of genes? => switch
Sure! Here’s a simpler explanation:
- Lac Repressor: This is a protein in bacteria that controls whether lactose-digesting proteins are made.
- Role of the Repressor: When there’s no lactose around, the lac repressor attaches to the bacteria’s DNA and stops the genes needed to break down lactose from being turned on.
- Lactose Present: When lactose is present, it binds to the lac repressor. This changes the shape of the repressor so it can’t stick to the DNA anymore.
- Gene Expression: With the repressor off the DNA, the bacteria can now produce the proteins needed to break down lactose.
So, basically, the lac repressor acts like a switch: it keeps lactose-processing genes off when there’s no lactose, and turns them on when lactose is available.

What are the 4 levels of protein structure?
- Primary structure – the sequence of amino acids
- Secondary structure – Local folded structures that form in a polypeptide due to interactions between atoms in the backbone of the structure. It consists of alpha helices, beta sheets and bends/loops
- Tertiary structure – The unique 3-dimensional folding of the structure. Proteins made from a single polypeptide only have up to tertiary structure
- Quaternary structure – How proteins consisting of multiple subunits come together

What is the general structure of amino acids?
What is unique to each amino acid?
What does this determine?
- Amino acids have an alpha carbon at the centre, an amino group to the right, a Hydrogen on the top, a carboxyl group on the left, and an R group on the bottom.
- The R- group is unique to each of the 20 amino acids, and its chemical properties determine the structure and function of the protein.

What are the 3 classifications of Hydrophilic amino acids?
What are features of each?
1 - 2 => ph
3 = bi
- Basic amino acids – positively charged R group, tend to contain NH groups
- Acidic amino acids – negative R groups, contain negatively charged carboxyl group
- Polar amino acids with uncharged R group – tend to contain OH or NH.

What are features of Hydrophobic amino acids?
ex = alaval
- Tend to contain long chains of Hydrocarbons

What are the 3 special amino acids?
prol
gly
cyst
Why are they considered special?
What is their structural formula?
1 shhhh
2 basic
3 chain
- Cysteine – can form disulphide (S-S) bonds with other cysteine residues
- Glycine – the smallest amino acid residue and can fit in tight spaces.
- Proline – a Carbon from Prolines R group bends round to form a covalent bond with the Nitrogen of the amino group. By doing this Proline creates a kink in the protein chain.

What are the structural formulas for Cysteine, Glycine, Proline, Histidine and Aspartate?
4 = recognise
5 = repartition


What is an acid and a base?
How do proteins exist at low vs high pH?
What is the pKa of an acid?
- An acid is any molecule that tends to release a hydrogen ion and a base is any molecule that readily combines with a hydrogen ion
- Proteins exist on protonated states at low pHs and deprotonated states at high pHs in an equilibrium
- The pKa of any acid is equal to the pH at which half of the molecules are disassociated (half in low pH form, half in high pH form.

what happens to proteins at diff pH
what varies in the protein with Ph
What is PKA
- The overall charge of an amino acid (and therefore the protein) varies with pH
- The pKa is the pH at which disassociation is 50% complete.
- This means at different pHs, the protein can alter in shape, which can prevent it from performing its function.
How is pKa/ pH involved in the uptake / degradation receptor-mediated endocytosis of LDL?
what a.a. is present in LDL
What is the condition associated with mutation of aminos present in the LDL receptor?
- The pH of the body is around 7.4
- Histidine residues are present in the LDL receptor, which has a pKa value of 6.5
- This means below pH of 6.5, Histidine mostly exists in its protonated form, and above a pH of 6.5, it mostly exists in its deprotonated form.
- When the LDL Receptor carrying LDL goes to the endosome as part of the uptake and degradation pathway, it changes shape due to the endosome having a lower pH of 5.
- LDL can no longer bind to the receptor and so is then released to the lysosome, while the LDL receptor its recycled and returns back to its original shape when it goes back to the regular pH of 7.4
- Patients with familial hypercholesterolemia frequently have mutations of the histidine residues in the LDL receptor

What is a polypeptide?
How are the R groups in a polypeptide arranged and why?
How do R-groups influence the folding of a polypeptide?
- Polypeptides are polymers of amino acids joined by peptide bonds.
- The R groups of amino acids are placed away from each other at the side of the chain, which allows the protein to adopt its lowest energy state.
- Non-polar (hydrophobic) R-groups are found on the inside of the protein and the Polar (hydrophilic) R groups are found on the inside of the protein when folding

What are peptide bonds? => opp ends
What kind of reactions are they formed in? => icey
- A peptide bond is a covalent bond formed when the carbon from the carboxylate group of 1 amino acid (red) shares electrons with the nitrogen atom from the amino group (blue) of another amino acid.
The carboxyl group (–COO⁻) of one amino acid reacts with the amino group (–NH₂) of another amino acid. - A molecule of water is lost, so this is called a condensation reaction.

What is the conformation of a protein?
How is the conformation of a protein limited by the peptide bonds?
How is it affected by R groups?
What determines the conformation of the protein?
- The conformation of a protein is the arrangement of its constituent atoms that determine the overall shape of the molecule.
- The peptide bond (grey shade) does not permit rotation.
- This is due to the C-O and C-N bonds in the peptide bond being partial double bonds.
- Bulky R-groups are positioned on either side of the backbone, which limits the number of 3D conformations possible for a polypeptide.
- There is rotation around the alpha carbon, meaning the conformation of the polypeptide is determined by one pair of angles for each amino acid residue.

What is the secondary structure of proteins?
What are the 3 types of secondary structures of proteins?
- Local folded structures that form in a polypeptide due to interactions between atoms in the backbone of the structure.
- It consists of alpha helices, beta sheets and bends/loops
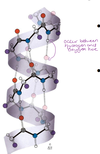
What is the structure of the α helix? What is it stabilised by? HAC
- The αhelix is right-handed (rotates in clockwise direction) and each turn has 3.6 amino acid residues
- The helix is stabilised by Hydrogen bonds between amino and carboxyl groups of every 4th amino acid.
What is the structure of β sheets?
What are the 3 types?
NCT
How does hydrogen bonding compare in each type?
- Extended stretches of amino acids are called β-strands
- Beta strands organised next to each other make beta sheets.
- There are parallel and anti-parallel beta sheet.
- If adjacent strands are oriented in the same direction (N to C), it is parallel, if adjacent strands run in opposite direction, they are anti-parallel.
- There can also be mixed beta sheets.
- Hydrogen bonding varies depending on the type of sheet
- Anti-parallel sheets are slightly more stable due to the hydrogen bonds being closer.

What are the structures of Bends/loops? What do they frequently contain?
- Polypeptide chains can fold upon themselves, forming a bend or loop
- Usually, 4 amino acids required to form the turn.
- Proline residues frequently found in the bend/loop as their kink in the chain helps to promoted bends/loops

What are hydrogen bonds?
When are they formed?
- Hydrogen bonds are intermolecular forces that form between the H in one molecule and the F, N or O of another molecule.
- Can only form if the Hydrogen is connected to an F, N or O in its own molecule.




