Unit 2: The Macromolecules Flashcards
Formula for
Glucose
C6H12O6
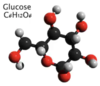
Monomer of
Carbohydrates
Monosaccharides
Types of
Carbohydrates
Monosaccharides
Disaccharides
Polysaccharides

Examples of
Monosaccharides
Glucose
Ribose
Galactose
Fructose
Other trioses, pentoses, and hexoses
Examples of
Disaccharides
Lactose
Sucrose
Examples of
Polysaccharides
Starch
Cellulose
Glycogen
Chitin
(You do not need to memorize the chart!)

What is/are
Glucose
Most important monosaccharide
C6H12O6<br></br>Used for immediate energy

What is/are
Disaccharides
Two monosaccharides bonded together

What is/are
Polysaccharides
Many monosaccharides bonded together
What is/are
Lactose
A disaccharide
Found in milk
What is/are
Starch
Polysaccharide
Plant glucose storage
Common in potatoes

What is/are
Glycogen
Polysaccharide
Animal glucose storage
Often stored in the liver or muscles

What is/are
Cellulose
Polysaccharide
Used by plants for forming the cell wall
Parts of
Fats and Oils
Glycerol and three fatty acids

Define
Triacylglycerol
A glycerol covalently bonded to three fatty acids
Fats and oils

Common feature of
Lipids
Hydrophobic, due to nonpolar bonds between carbons and hydrogens
Examples of
Lipids
Fats and oils
Waxes
Phospholipids
Cholesterol and steroids
Pigments
Define
Saturated fatty acid
A hydrocarbon with all single bonds
Each carbon in the chain is bound to two hydrogen atoms

Define
Unsaturated fatty acid
A hydrocarbon with one or more double bonds
Some of the carbon atoms in the chain are bound to only one hydrogen atom

Define
Fat
Triacylglycerides with all saturated fatty acids or trans-fatty acids, which can pack in tightly to form a solid
Define
Oil
Triacylglycerides with some cis-unsaturated fatty acids, which do not pack in tightly enough to form a solid
Define
Cis-fatty acid
Fatty acid chain with a double bond in the cis configuration
The molecule is bent

Define
Trans-fatty acid
Fatty acid chain with a double bond in the trans configuration
The molecule can be in a mostly-linear structure
Found mainly in foods that have been chemically processed

Define
Steroid
Lipids that have a four-fused-ring structure
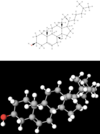
List types of
Steroids
Cholesterol Hormone molecules (ex: testosterone, estrogen, and progesterone)
Monomer of
Proteins
Amino acids

Structure of
Amino acids
A central carbon attached to:
- A hydrogen atom
- A carboxyl group
- An amino group
- An R group

Define
R group
The part of an amino acid that differs to make each amino acid unique

Define
Polypeptide
A strand of amino acids

Define
Primary structure
The sequence of amino acids in a protein

Define
Secondary structure
The localized folding patterns of a polypeptide

Define
Tertiary structure
The overall folding pattern of a polypeptide

Define
Quaternary structure
The joining of two or more polypeptides and/or other molecules to form a functional protein

What causes
Primary structure
The DNA instructions
What causes
Secondary structure
Hydrogen bonding between amino groups and carboxyl groups of amino acids (not adjacent to each other)
What causes
Tertiary structure
Chemical attraction of R groups within a polypeptide

What causes
Quaternary structure
Chemical attraction of R groups of different polypeptides
Examples of
Secondary structure
β- pleated sheets
α-helix
Examples of
Tertiary structure
Ionic bonding
Disulfide bridges
Hydrogen bonding
Hydrophobic interactions
Functions of
Proteins
Enzymes
Transport
Structure
Hormone
Immunity
Movement
Storage
Define
Denaturation
The loss of the shape of a molecule (usually proteins)

Causes of
Denaturation
Changes in temperature, pH, or other conditions that lead to weakened chemical attraction of parts of the polypeptide, causing it to lose its three-dimensional shape (and therefore its function)
Monomer of
Nucleic acids
Nucleotides
Types of
Nucleic acids
DNA and RNA
Describe the function of
DNA
Carry the genetic instructions for building proteins
Passed on to new cells during cell division / reproduction
Describe the function of
RNA
Involved in using the DNA “code” to build proteins
List the parts of
Nucleotides
Phosphate group
Five-carbon sugar
Nitrogenous base

List the
Types of nitrogenous bases
Purines: Adenine and guanine
Pyrimidines: Cytosine, thymine, and uracil

Describe
DNA nucleotides
Phosphate, deoxyribose, and either A, C, T, or G
Describe
RNA nucleotides
Phosphate, ribose, and either A, C, U, or G
Describe the structure of
DNA
Sugar-phosphate backbone with nitrogenous bases facing into helix
Hydrogen bonds form between thymine and adenine or guanine and cytosine
Double helix coils into chromosomes

Numbering system in
Nucleotides
1’ carbon binds to nitrogenous base
3’ carbon binds to free hydroxyl group
5’ carbon binds to phosphate group

Define
Antiparallel
In DNA double helix, the backbone runs in opposite directions
One strand runs 5’->3’ and the other runs 3’->5’



