Tavalin - Rxs for Mvmt Disorders Flashcards
(28 cards)
What is the difference bt parkinsonism and parkinson’s disease?
- Parkinsonism = unknown cause/idiopathic (environmental, trauma, etc.)
- Parkinson’s disease = familial
- REMEMBER: case in class about soccer player with parkinsonism symptoms at age 40
What blockade of dopamine receptors be an effective pharm strategy for PD mgmt?
NO
Although metabolic enzymes are important targets for tx of PD, name a PD drug that has a target that lacks direct metabolic activity.
Amantadine (antiviral drug)
What is one of the medical strategies for the mgmt of early HD?
D2 antagonists
37-y/o woman with tremor who becomes combative and bottle of pills falls out of her pocket.
Is she more likely to have PD or parkinsonism? What pills is she likely to have?
- Familial Parkinson’s because early onset
- Ropinirole -> don’t start Levodopa/Carbidopa until pts have a disability/advanced disease
63-y/o male farmer with borderline HTN referred due to 1-year non-disabling intermittent resting tremor of left hand that later progressed to contralateral hand. On neuro exam, pt had normal cognition. Intermittent mild resting tremor observed in left hand, and mild signs of asymmetrical cogwheel rigidity and bradykinesia. Gait and balance were normal, as well as postural reflexes.
Dx? Med?
- Parkinson’s disease
- Pramipexole to manage progression of disease at current stage
Case with older man with progressing Parkinson’s. What is best tx option for DEC his time off?
Add Entecapone to his regimen
What agents are used for the tx of PD?
- DA replacement tx: L-dopa, L-dopa/carbidopa
- DA receptor agonists: Bromocriptine, Pramipexole, Ropinirole, Apomorphine
- Enhancement of DA release: Amantidine
- INH of DA metabolism: MAO-B (Selegiline, Rasagiline), COMT (Entacapone, Tolcapone)
- Antimuscarinics: Benztropine, Diphenhydramine, Trihexyphenidyl
What agents are used in the tx of HD?
- VMAT INH (DA-depleting agents): Reserpine, Tetrabenazine
- Dopamine D2 receptor antagonists: Chlorpromazine, Haloperidol
What is PD? 4 main clinical features?
- Progressive mvmt disorder with avg age of onset of 55 (about 10% of cases under 40)
- 4 main clinical features:
1. Bradykinesia: slowness of mvmt
2. Muscular rigidity: INC mm tone, and resistance to mvmt
3. Resting tremor: abates during voluntary mvmt
4. Postural instability and impaired gate
What is the pathophys of PD and parkinsonism?
- IDIOPATHIC PD: unknown origin, but exposure to neurotoxin or generation of free radicals thought to contribute
1. Evidence for genetic component: AUTO DOM forms of disease in muts of genes encoding alpha-synuclein (synaptic protein), LRRK2 (leucine-rich repeat kinase 2), parkin (ubiquitin hydrolase), and UCHL1 (also involved in ubiquitin-mediated protein degradation) - PARKINSONISM: associated w/envo factors like infection w/encephalitis, stroke, trauma, antipsychotic drug tx, MPTP neurotoxin (often tx-resistant)
- NOTE: hallmark feature of either is selective loss of pigmented (neuromelanin) neurons in substantia nigra pars compacta -> most symptoms do not occur until striatal DA neuron levels decline by at least 70-80%
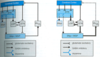
Briefly describe the basal ganglia and its 2 pathways.
- Several lg, subcortical nuclei that participate in control of mvmt -> 2 pathways that form feedback loop to cortex
- DIRECT: cerebral cortex -> striatum (SNpc) -> SNpr/GPi -> thalamus (excitatory drive) -> cortex w/net effect of INC thalamic output
- INDIRECT: cortext -> striatum (SNpc) -> GPe -> sub-thalamic nucleus -> GPi SNpr -> thalamus (excitatory drive) -> cortex w/net effect of DEC thalamic output
- Primary input from cerebral cortex, and output directed through thalamus back to prefrontal, premotor, and motor cortex
Under normal conditions, does the SNpc favor the direct or indirect pathway? How is this mediated?
- SNpc activates striatal neurons that project to direct pathway via D1 receptors
- In contrast, striatal neurons that project to indirect pathway are INH by D2 receptors
- Under normal conditions, SNpc favors DIRECT PATH over the indirect pathway -> in PD, activity of indirect pathway predominates (due to loss of dopaminergic output from SNpc)
- NOTE: D2 receptor activation appears to be most important in gating balance of the 2 pathways

Note the predominance of the indirect pathway in PD (right image).
Good job!
What are the 5 main strategies for PD pharmacotherapy? Rationale?
- Replace DA
- Stimulate DA receptors
- Enhance DA release
- INH DA metabolism
- Alter DA/Ach balance
- RATIONALE: PD characterized by loss of DAergic neurons in SNpc
1. Drugs that destroy DAergic neurons cause P
2. Drugs that block DA receptors (i.e., anti-psychotics) can cause P
3. Cholinergic neurons in striatum enhance GABAergic output

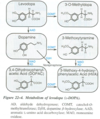
Describe the MOA and pharmacokinetics of DA replacement therapy for PD.
- DA cannot cross BBB into CNS, so L-dopa, the immediate precursor for dopamine, is the single most effective tx for PD -> can completely ameliorate all symptoms of PD, esp. during initial tx
- MOA: replenishes DA stores in remaining DA terminals in striatum
- PHARMACOKINETICS: readily absorbed in GI tract (dependent on gastric emptying); plasma conc peak fast (1-2 hrs) and 1/2-life brief (1-3 hrs)
1. Converted in brain and periphery to DA by L-aromatic amino acid decarboxylase (L-AAD)
2. 99% of systemically admin’d L-dopa converted to DA in periphery, and excreted in urine as HVA and DOPAC -> L-dopa must be given in high doses when admin’d alone - Co-administered w/Carbidopa (L-AAD INH) that can’t cross BBB -> prolongs L-dopa 1/2-life, DEC amt that needs to be admin’d by 75%, and DEC side effects due to DEC level of peripheral DA
What are the AE’s of DA replacement therapy in PD?
- Predominantly in response to L-dopa monotherapy:
1. GI (anorexia, N/V) tend to DEC w/repeated use
2. CV (arrhythmias-tachy, ventricular extra-systoles, afibs): incidence low, except in those predisposed; orthostatic hypoTN - Predominantly in reponse to combo tx w/Carbidopa:
1. Behavioral (depression, anxiety, delusion, agitation, insomnia, hallucinations, nightmares, euphoria, and o/alterations in mood/personality); use anti-psychotics
2. Dyskinesias (chorea, myoclonus, tics, tremor -> non-resting, intentional) - Fluctuations in response: “on-off” phenomenon
What are the drug interactions and contraindications to DA replacement therapy for PD?
- DRUG INTERAXNS: Vit B6 (pyridoxine) INC L-dopa metabolism, so need decarboxylase INH (Carbidopa)
1. Should NOT be given to pts on MAO-A INH due to HTN crises
2. Give b4 meals due to competition w/L-AA’s in food for transporters in GI tract - CONTRA: psychotic pts, glaucoma (angle-closure), cardiac disease, peptic ulcer, melanoma
- NOTE: tx w/L-dopa often effective for 3-5 yrs, so often delayed until symptoms of PD yield functional impairment
1. While controversial, also thought L-dopa may exacerbate progression of PD due to generation of free radicals
What is the rationale, and what are the advantages of DA agonist tx for PD? What agents are currently in use?
- Rationale to principally activate D2 receptors to DEC activation of indirect pathway -> preferred tx for early PD (and may be used in combo w/L-dopa in advanced disease to reduce “on-off”)
- ADVANTAGES: do not need to be converted to active compound, no potentially toxic metabolites, do not compete w/o/things for GI absorption or across BBB -> may be more selective, thereby DEC AE’s
- Agonists in current use:
1. Bromocriptine (D2 agonist/D1 partial agonist)
2. Apomorphine (D1/D2 agonist)
3. Pramipexole (D2 selective; may also act as free radical scavenger)
4. Ropinirole (D2 selective; metabolized by CYP1A2)
What are the MOA, pharmacokinetics, and AE’s of Amantidine?
- Antiviral agent that alleviates parkinsonian symptoms
- MOA: unclear, but appears to enhance release and possibly DA synthesis; may also INH DA uptake
1. Possible interaxn w/NMDA receptors
2. Effects modest, and short-lived - PHARMACOKINETICS: peak plasma conc (1-4 hrs), 1/2-life (2-4 hrs), excreted primarily in urine
- AE’s: restlessness, depression, agitation, irritability, insomnia, excitement, hallucinations, confusion -> OD may produce psychosis
1. Other AE’s similar to those produced by L-dopa and DA agonists
2. CONTRA in pts w/hx of seizures/heart failure
What is the MOA of Selegiline? Indications? Interactions? AE’s?
- SELEGILINE: selective MAO-B INH that retards breakdown of DA
1. Metabolites incl amphetamine/meth -> INC DA release; may be neuroprotective and DEC PD progression by INH MAO-B-mediated formation of free radicals - INDICATIONS: used 1o in pts whose responsiveness to L-dopa has declined; little effect alone
- INTERAXNS: do NOT take w/Meperidine, TCA’s, or SSRI’s
- AE’s: may potentiate AE’s of L-dopa
- NOTE: RASAGILINE a new, more potent MAO-B INH approved for combo tx w/L-dopa in late-stage PD, or alone in early PD
What are the MOA, indications, and pharmacokinetics of Entacapone/Tolcapone? Differences bt the 2 and preferred tx?
- MOA: selective INH of COMT -> prolong action of L-dopa, DEC production of 3-OMD, which may compete w/L-dopa for transport carriers in GI and BBB
1. INC bioavailability of L-dopa - INDICATIONS: DEC fluctuation responses to L-dopa
- PHARMACOKINETICS: rapidly absorbed, highly protein-bound (1/2-life about 2 hrs)
- TOLCAPONE: central and peripheral effects (Entecapone strictly peripheral)
1. ENTECAPONE: preferred bc Tolcapone may INC liver enzymes/cause liver failure (requires signed pt consent)
How are muscarinic antagonists used to tx PD? Which ones? AE’s?
- MOA: block cholinergic activation in striatum
1. Benztropine, Diphenhydramine, Trihexyphenidyl - Widely used prior to L-dopa advent; used in early PD, or as adjunct to L-dopa
- AE’s: drowsiness, mental slowness/inattention, confusion, delusions, hallucinations, mood changes
1. Other common effects characteristic of anti-cholinergic rxs: dry mouth, blurry vision, urinary retention, constipation, tachy, arrhythmias, INC intraocular pressure, palpitations, N/V, etc. - CONTRA: prostatic hyperplasia, OBD (opioid bowel disease), glaucoma; avoid concomitant use of drugs w/anti-musc effects (i.e., TCA’s or anti-histamines)
Describe a future PD tx target.
- L-type Ca2+ channel antagonists
- Underly pacemaking activity in substantia nigra dopamine neurons, leading to continual influx of Ca, and INC susceptibility to 2o insults
- When blocked by ISRADIPINE, pacemaking reverts to HCN1 and Na channel-dependent pacemaking, which occurs in juvenile neurons
- This REJUVENATION may prevent disease progression


