Reversible Reactions and Equilibrium Flashcards
What is the symbol for a reversible reaction
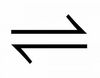
What is the definition of Dynamic Equilbirium?
When a reversible reaction takes place in a sealed container and the forward and reverse reactions occur at the same rate
What are the 2 conditions that we can change that will affect the direction of the reaction?
Temperature Pressure (of gases only)
What does the system try to do when we change temperature of the system (reaction)?
The system (reaction) responds to counteract the change. eg increase temp leads to increase in endothermic reaction eg decrease temp leads to increase in exothermic reaction
What does the system try to do when we change pressure of gases?
The system (reaction) responds to counteract the change. eg increase pressure leads to increase the reaction that has less molecules eg decrease pressure leads to increase the reaction that has more molecules
The Haber Proess adds two gases to make ammonia in a reversible reaction
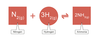
This is an example of a reversible reaction between Ammonia and Hydrochloric acid

Does adding a catalyst affect equilbirum?
No


