Immuno 3 Flashcards
What is the influenza virus and what symptoms does it create?
- RNA virus that infects the respiratory tract
- productive cough (excess mucus, cilia, expulsive reflexes) *intrinsic barriers
- generalized aches (inflammatory mediators eg. prostaglandin)
- fever (inflammatory mediators eg. IL-1, IL-6, TNF-alpha)
When influenza arrives, what is the first thing it will encounter in the immune system?
- resident defences
- cells detect influenza with with TLR 3 (dsRNA) and other PRRs
- epithelial cells and macrophages produce interferons (antiviral cytokines) and cytokines/chemokines
- after seeing PAMP, dendritic cells mature, process, and present influenza peptides in MHC 1 (if infected themselves) or MHC 2 (after phagocytosing material)
What changes occur when a dendritic cell matures?
- they express different proteins on the surface
- B 7.1 and B 7.2 are expressed in mature dendritic cell
- ICAM-1 and LFA-1; adhesion molecules that allow dendritic cell out of tissue and provide costimulation to T cells when they find them
- CCR7 is a receptor for chemokines; allows dendritic cell to find its way to the lymph node
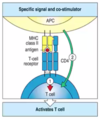
When the dendritic cell or APC arrives in the lymph node, what occurs?
- activated dendritic cells present influenza peptides in MHC to many naive T cells (this is why lymph node exists- area of high density of T and B cells)
- when T cell with a TCR specific for influenza virus finds that dendritic cell, they couple and the T cell becomes activated
- costimulatory receptors on surface of APC bind to receptors on T cell to provide 2nd signal; these receptors are only found on mature dendritic cells
- double stranded RNA on influenza which activates the PRR on surface of APCs which causes the APC to mature to express costimulatory receptors so that when it arrives in the lymph node it can stimulate T cells that pass by
- the T cell divides rapidly and differentiates to become effector T cells
How does the T cell interact with the B cell?
- activated T cell runs into many B cells in lymph node
- if activated influenza specific T cell finds influenza specific B cell it can activate the B cell
- first signal is influenza binding to the B cell receptor
- T cell provides costimulation with cytokines, chemokines, and growth factors that allow the B cell to differentiate and divide rapidly to become a memory or plasma B cell
- B cell enters germinal centre to divide

What do CD4 helper T cells do?
- respond to MHC 2 and influenza peptide
- produce cytokines (interferon- anti viral cytokines) to activate macrophages and other cells
- provide help to B cells to activate them and make them into plasma or memory cells; activate B cells to produce antibody that can neutralize influenza virus
- T helper 1 cells bind to surfaces on macrophages and provide the stimulus the macrophage needs to kill the bacteria it has engulfed (eg. in tuberculosis, it is not killed until there is a signal to tell macrophage to kill it)
What do CD8 killer cells do?
- recognize influenza in context of MHC 1 and influenza peptide
- secretes perforin and granzyme (perforin makes hole in cell wall and granzyme is an enzyme that can go in and turn on the cell death cascade in the cell), fas-ligand (binds to fas on surface of infected cell to induce apoptosis), and other molecules that cause influenza infected cells to undergo apoptosis
- this prevents infection from spreading
What are the effector functions of B cells?
- Antibodies can neutralize free influenza virus; influenza needs to bind to the surface of a cell to infect it so binding of antibodies prevents this by coating surface of virus
- Target cells which are infected by influenza virus; antibodies alone aren’t enough to kill a host cell but they can mark the surface of the cell for a NK cell to recognize
- the NK cell has receptors that will bind to the constant domain of the antibody which allows the NK cell to find infected cells (NK has no natural homing abilities)
- NK cell degranulates and causes infected cell to undergo apoptosis - Antibodies activate complement; activation of inflammation (dilation and permeability of blood vessels and recruitment of proteins, antibodies, immune cells into infected area)
- Antibodies are critical to influenza-specific immune memory- vaccines are based in long lived antibodies and B cells to prevent against future attacks
How is the primary immune response regulated?
- immune responses are self-limiting
- when T cell is activated, first signal comes from TCR then second signal is provided by B7 binding to CD28, a costimulatory receptor on the surface of the T cell
- this causes it to differentiate and divide to conduct effector functions
- CTLA-4 begins to express on surface of cell while CD28 is bound to B7
- CTLA4 also binds to B7 more strongly than CD28 so it is able to displace CD28
- CTLA4 provides inhibitory signal to the T cell so anytime a T cell is activated, one of the first things it does is produce CTLA 4 to turn it off

Describe the activation, expansion, contraction, and memory phases of primary immune response
- activation: most of the T cells are not specific to the antigen at the beginning of the response. If an antigen-specific T cell is able to find the antigen, expansion occurs:
- expansion: large growth in antigen specific T cells
- contraction: as CTLA 4 begins to be expressed and as that provides a negative signal to the T cells, contraction phase ensues and antigen specific T cells begin to die
- memory: left with more antigen specific t cells than we did at the activation phase
- advantage of the contraction phase is that the best-binding and strongest T cells are the ones that will survive

What are the functions of immune memory within a secondary immune response?
- protects against re-infection
- controls persistent infections; sometimes a virus can’t be cleared but the T cells and B cells can eventually control it where it causes less problems
- protects immunologically immature offspring from primary infections; IgG passed across placenta which protects baby in first 6 months of life- at this point baby does not have ability to mount a good primary immune response so they use mom’s secondary immune response in the meantime
What are other functions that the secondary immune system has?
- mucosal lining in lungs secretes antibodies into the lumen of the lung which are waiting there to neutralize influenza virus if it comes back before it can infect a pneumocyte to prevent reinfection of the same strain
- long lived memory T and B cells waiting and are able to rapidly divide and provide additional protection if antibodies in circulation aren’t enough; faster than primary immune response, more effective and more potent because the weakest T and B cells are weeded out in contraction phase so only the best make it to become memory cells
- only pass antibodies across the placenta, not B and T cells
- gives extremely long lived protection; these cells survive a long time without needing a stimulus to keep them there (eg. smallpox specific T cells can still be detected in the blood of those were exposed a long time ago)
What is autoimmunity?
- not all autoreactive T and B cells are removed during negative selection, and can attack “self”
- eg. rheumatoid arthritis: B and T cells are attacking self proteins
What is immunopathology?
- some immune responses are inappropriately robust and can hurt us by accident
- we are injured as a bystander (target isn’t self)
- ARDS: immune system is trying to clear an infection in lungs but there is so much inflammation in lungs that people are unable to breathe and can die because their immune system has affected lung function
What is an allergy?
- inappropriate immune response to a non-self protein that most people ignore
- breakdown in tolerance leads to immune response against harmless environmental antigens
- eosinophils move to the site of allergy and make inflammatory mediators and respond to cytokines
- eosinophils and other cells produce cytokines which get the beta cells to make IgE antibodies
- beta cells make IgE antibodies against the allergen, which coats mast cells, and when the allergen is introduced they respond predictably
What idea did Janeway present regarding how the immune system response is activated and what did Matzinger add to it?
- not so much that it is self vs non self that tells the immune system to respond to it
- instead, if something has a PAMP with it it will be attacked
- if something is non self the immune system will attack it because it has a PAMP
- if something turns on the innate immune system, the adaptive will see it as a pathogen and attack it
- limits to this is that our immune system will attack our own proteins even though there is no identifiable pathogen in the area
- Matzinger described idea of DAMPS: danger associated molecular patterns
- DAMP hypothesis is that it is not specifically pathogen molecules like LPS, double stranded RNA but anything which will bind to a danger receptor (PRR or something that picks up endogenous tissue damage or other signs of tissue damage)
- if damage is being done to tissues, immune system should get involved and try to clear what is doing the damage
Describe the current knowledge given that there is no grand theory about self vs non self
- APCs present lots of antigens to T cells (mostly innocuous “self” antigen; most APCs have proteins in MHC1 and 2 which are self proteins which should not be attack target for immune system)
- most APCs are in an inactive state and not by nature activating T cells, only when there is a danger in the form of PAMPs or DAMPs
- APC express costimulatory molecules to prime T cells under these conditions
- Whatever APCs are presenting at the time is a potential immunogen, whether self or non self
- if an APC picks up one of our own proteins at the same time it ran into influenza virus infection, influenza will be on the surface with our own endogenous proteins
- that cell is now activated so it will go to lymph node and display self and influenza to the T cells
- T cells will see protein in MHC on activated APC and if it happens to be specific for one of our own proteins, then that T cell presumably will become activated and will attack our own cells
What mechanisms are in place to prevent attack of “self”
- negative selection (central tolerance) where T cells are screened against self proteins
- regulatory T cells: special subset of CD4 T cells which have the ability to suppress dendritic cells and other T cells, act as a break on the system by secreting anti-inflammatory cytokines to dampen immune responses and prevent autoimmunity/immunopathology
- anergization/clonal deletion: rely on T cells needing 2 signals to become activated, if a T cell ever sees antigen in MHC and it binds to it but there is no costimulatory signal in the area that tells T cell it is not dangerous, causes T cell to undergo anergization where cell becomes inert
- if that T cell runs into an APC in the future with its antigen, even if that APC is activated and expressing costimulatory molecules that T cell won’t respond
- this presumably would happen on an APC that has self proteins on its surface as well as influenza- only reason that is an activated APC is not because the self protein is the threat but because the self protein is on the same surface as an activated APC that has an unrelated threat as well such as influenza
What is the target in systemic lupus erythematosus?
-immune response is against nuclear antigens
What is the target in multiple sclerosis?
-myelinating oligodendricytes
What is the target in rheumatoid arthritis?
-collagen
What is the target in pemphigus vulgaris?
-desmoglein
What is the target in myasthenia gravis?
-acetylcholine receptors
What is the target in insulin dependent type 1 diabetes?
-pancreatic beta cells




