Energetics & Equilibria 5: Electrochemistry Flashcards
Cell conventions:
- Each half cell is written as a reduction
- Both half cells involve the same #electrons, so conventional cell reaction (NB not necessarily spontaneous) = RHS half-reaction - LHS half-reaction
How is the cell potential calculated from the half-cells?
Ecell = RHS half-cell potential - LHS half-cell potential
Describe the conventional shorthand notation for cells.
, indicates species in the same solution in the same phase
|| (or | x |) indicates a liquid junction (junction between solutions)
indicates species in the same solution in different phases
For the following reaction, write
- the half-cells
- the shorthand notation for the cell
Cu2+(aq) + Zn(m) –> Cu(m) + Zn2+(aq)
Conventional cell reaction = RHS - LHS, so:
LHS: Cu2+(aq) + 2e- –> Cu(m)
RHS: Zn2+(aq) + 2e- –> Zn(m)
Cu(m) | Cu2+(aq) || Zn2+(aq) | Zn(m)
One | indicates different phases, and || indicates a liquid junction (junction between solutions)
Write the conventional cell reaction for the following cell:
Pt(m) | H2(g) | H+(aq) || Cd2+(aq) | Cd(m)

Give the equation relating cell potential (of the conventional cell reaction) to Gibbs energy.
Cell potential is emf. NB proof for this equation is not required.
ΔrGcell = -nFE (constant temp and pressure)
Where F = Faraday constant, i.e. the charge on one mole of fundamental charges, and n = #electrons involved in cell reaction
I.e. in cells, emf is related to change in gibbs energy - allowing direct measurement. of gibbs energy
Derive an expression for the entropy change of a cell reaction.
Recall 3rd master equation dG = Vdp - SdT
So dG = -SdT (constant pressure)
S = -dG/dT (constant pressure)
S = -(∂G/∂T)p
Apply this to a cell:
ΔrS = -(∂ΔrGcell/∂T)p
ΔrGcell = -nFE
ΔrS = -(∂(-nFE)/∂T)p
= nF(∂E)/∂T)p
I.e. practically, measure cell potential E over a range of temperatures, then plot E against T, and the entropy change is the slope.
How is the enthalpy change of a cell calculated?
Rearrange ΔrGcell = ΔrHcell - TΔrScell
Find ΔrGcell using ΔrGcell = -nFE
Find ΔrScell using ΔrScell = nF(∂E)/∂T)p
So ΔrHcell = TnF(∂E)/∂T)p - nFE
Write the expressions for:
- The chemical potential of a gas
- The chemical potential of an ideal solution
- (Recall for solids/liquids chemical potential = standard chemical potential)
Gas: µi(pi) = µio + RTln(pi/po)
Ideal solution: µi(ci) = µio + RTln(ci/co)
For an ideal solution, µi(ci) = µio + RTln(ci/co)
However, solutions of ions are rarely ideal, so chemical potential must instead be written in terms of a different quantity. Write this expression.
activity, ai:
µi(ai) = µio + RTln(ai)
Where, as ci –> 0, ai = (ci/co)
i.e. solution becomes ideal in the limit of low concentration
Write the Nernst equation for the generic reaction of solutions:
VAA + VBB –> VPP + VQQ
E = Eo - (RT/nF)ln( ((ap)Vp(aQ)VQ) / (aA)VA(aB)VB) )
Generally, E = Eo - (RT/nF) ln(products/reactants)
Derive the Nernst equation for the generic reaction of solutions:
VAA + VBB –> VPP + VQQ
ΔrG = VpµP + VQµq - VAµA - VBµB
Recall, for non-ideal species i, µi(ai) = µoi + RTln(ai)
- Sub in expressions for each activity in above equation
- gather up the Viµi terms into square brackets, note they = ΔrGo, so replace them with this
- Gather the ln terms, raising the stoichiometric coefficients
Then follow image
E = Eo - (RT/nF)ln( ((ap)Vp(aQ)VQ) / (aA)VA(aB)VB) )

Consider the reaction VAA + VBB –> VPP + VQQ
When the species are all solutions, the Nernst equation is:
E = Eo - (RT/nF)ln( ((ap)Vp(aQ)VQ) / (aA)VA(aB)VB) )
What is the equation when all species are gases?
E = Eo - (RT/nF)ln( ((pP/po)Vp(pQ/po)VQ) / (pA/po)VA(pB/po)VB) )
Replace activities with (partial pressure/standard pressure)
Consider the reaction VAA + VBB –> VPP + VQQ
When the species are all solutions, the Nernst equation is:
E = Eo - (RT/nF)ln( ((ap)Vp(aQ)VQ) / (aA)VA(aB)VB) )
What is the equation when all species are solids/liquids?
E = Eo - (RT/nF)ln(1)
So E = Eo
Recall chemical potential = standard chemical potential
- Give the generic form of the Nernst equation for half-cells
- Give the equation linking standard half-cell potentials to the standard cell potential
E1/2 = Eo1/2 - (RT/nF) ln(products/reactants)
Eo = Eo1/2(RHS) - Eo1/2(LHS)
Define the standard half-cell potential of an electrode.
The potential of a cell in which the left-hand electrode is the standard hydrogen electrode, and the right-hand one is the one under test, and all species are present at activity = 1.
For the standard hydrogen electrode:
- Write the conventional notation for this cell
- Write the half-cell reaction
- Describe the organisation of the cell
Pt(m) | H2(g, p = 1 bar) | H+(aq, a = 1)
H+(aq) + e- –> 1/2H2(g)
H2(g) at p = 1 bar in contact with H+(aq) at activity = 1, where an inert Pt electrode makes the electrical contact.
The spontaneous cell reaction isn’t necessarily the conventional one, rather it’s the one which would occur if current were to flow.
State how the sign of the cell potential indicates whether the conventional cell reaction is spontaneous.
If cell potential E is positive, ΔrGcell is negative, since ΔrGcell = -nFE. So the direction of the conventional cell reaction is spontaneous.
If cell potential E is negative, ΔrGcell is positive, since ΔrGcell = -nFE. So the opposite direction of the conventional cell reaction is spontaneous.
How could you make a non-spontaneous conventional cell reaction spontaneous?
Alter the concentrations of ions, since cell potential E is concentration-dependent according to the Nernst equation.
The following is a metal/metal ion half-cell: a metal in contact with a solution of its ions. Write the Nerst equation for it.
Ag+(aq) + e- –> Ag(m)
E = Eo(Ag+, Ag) - (RT/F) ln(1/aAg+)
note n = 1 here so RT/nF = RT/F
Describe gas/ion half-cells
A gas in contact with a solution of related ions, where an inert Pt electrode provides the electrical contact
e.g. chlorine gas forms Cl-
A gas/ion half-cell involves H2(g) and OH-(aq). Write the conventional cell reaction and the Nernst equation.
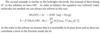
Describe redox half-cells.
Oxidised and reduced species are present in solution, where an inert Pt electrode provides electrical contact
E.g. Fe2+/Fe3+
Describe metal/insoluble salt/anion half-cells.
An anion is in solution, and a metal is coated with a layer of the insoluble salt formed by the metal and anion.

Example image
When the solutions in the two half-cells are different, they must be in contact without mixing.
- A liquid junction provides a porous barrier, allowing contact without rapid mixing. What is the issue with this?
- How do salt bridges avert this issue?
- A liquid junction potential may establish, which detracts from the cell potential, making the measurement inaccurate
- Salt bridges provide electrical contact whilst minimising liquid junction potential: tube typically containing conc KCl or KNO3, sealed by glass sinters at each end, which dips into both solutions


