Energetics & Equilibria 2: Gibbs energy Flashcards
- Definition of Gibbs energy, G (eq)
- Give its unit
- G = H - TS
- kJ mol-1 (since it’s a measure of energy)
this quantity allows you to just consider the system at hand, rather than the universe
What is true of G, for constant temp and pressure,
- during a spontaneous process?
- at eq?
Spontaneoous: ΔG < 0 (G decreases)
Eq: ΔG = 0 (G is at a mimimum)
Show that G decreases for a spontaneous process, and ΔG = 0 at equilibrium.
First consider G
Definition of G
G = Hsys - TsysSsys
Complete differential, then use product rule:
dGsys = dHsys + d(-TsysSsys) = dHsys - TsysdSsys - SsysdTsys
At constant temp, SsysdTsys = 0, so:
dGsys = dHsys -TsysdSsys (constant temp)
- Divide both sides by -T*
- dGsys/Tsys = -dHsys/Tsys + dSsys (constant temp)
Then consider entropy
Recall ΔSuniv = ΔSsurr + ΔSsys = -qsys/Tsurr + ΔSsys
Differential form:
dSuniv = - δqsys/Tsurr + dSsys
At constant pressure, δqsys = dHsys. And system and surroundings are at same temp, so:
dSuniv = - dHsys/Tsys + dSsys (constant pressure)
Then combine them (and their conditions)
-dGsys/T = dSuniv (constant temp and pressure)
Conclusions
- In a spontaneous process, Suniv increases, so dSuniv is positive, so, for constant temp, dGsys must be negative, so ΔG < 0. So G falls
- At equilibrium (reversible process), dSuniv = 0, so dSuniv = 0, so dGsys = 0, so ΔG = 0. So G is at a minimum
show how to get from
G = Hsys - TsysSsys
to
ΔG = ΔH - TΔS (constant temp and pressure)
Definition of G
G = Hsys - TsysSsys
Complete differential, then use product rule:
dGsys = dHsys + d(-TsysSsys) = dHsys - TsysdSsys - SsysdTsys
At constant temp, SsysdTsys = 0, so:
dGsys = dHsys -TsysdSsys (constant temp and pressure)
Write in terms of finite changes (rather than infinitesimal)
ΔGsys = ΔHsys - TΔSsys (constant temp and pressure)
Drop sys subscripts since it’s implied that we’re discussing system
ΔG = ΔH - TΔS (constant temp and pressure)
When is it true that dU = δq - pdV?
The only work done is that of gas expansion:
dU = δq - pdV (pV work only)
Give the first, second and third master equations
dU = TdS - pdV
dH = TdS + Vdp
dG = VdP - SdT
- Derive the first master equation:
dU = TdS - pdV
- Explain why it’s true for both reversible and irreversible processes
1.
dU = δq - δw
dU = δq - pdV (pV / gas expansion work only)
Assume reversible
dU = δqrev - pdV
dS = δqrev/T
δqrev = TdS
Combine eq
dU = TdS - pdV
2. True for all changes since U is a state function. When reversible, δq = TdS and δw = pdV. When irreversible, this isn’t true, but their stum still = dU.
Derive the second master equation:
dH = TdS + Vdp
Derive the first one:
dU = δq - δw
dU = δq - pdV (pV / gas expansion work only)
Assume reversible
dU = δqrev - pdV
dS = δqrev/T
δqrev = TdS
Combine eq
dU = TdS - pdV
Use this to derive the second
- Definition of enthalpy:* H = U + pV
- Complete differential, then product rule:*
dH = dU + d(pV) = dU + pdV + Vdp
Sub in first eq
dH = (TdS - pdV) + pdV + Vdp
dH = TdS + Vdp
Derive the third master equation:
dG = Vdp - SdT
Derive the first one:
dU = δq - δw
dU = δq - pdV (pV / gas expansion work only)
Assume reversible
dU = δqrev - pdV
dS = δqrev/T
δqrev = TdS
Combine eq
dU = TdS - pdV
Use this to derive the second
- Definition of enthalpy:* H = U + pV
- Complete differential, then product rule:*
dH = dU + d(pV) = dU + pdV + Vdp
Sub in first eq
dH = (TdS - pdV) + pdV + Vdp
dH = TdS + Vdp
Then derive the third
- Definition of G**:* G = H - TS
- Differential:* dG = dH - d(TS) = dH - TdS - SdT
- Sub in dH from 2nd eq*
dG = TdS + Vdp - TdS - SdT
dG = Vdp - SdT
Why is the 3rd master eq so useful?
dG = Vdp - SdT
change in pressure and temp can be used directly to calculate Gibbs energy
Give the expression for how molar Gibbs energy varies with pressure, at constant temp, for an ideal gas.
Gm(p) = Gom + RT ln(p/po) (const. temp, ideal gas)
Derive this expression for how molar Gibbs energy varies with pressure, at constant temp, for an ideal gas.
Gm(p) = Gom + RT ln(p/po) (const. temp, ideal gas)
- Third master eq:* dG = Vdp - SdT
- At constant temp, dT = 0, so*
dG = Vdp (const temp)
V varies with pressure p according to ideal gas eq:
pV = nRT, so V = nRT/p
Sub into eq for dG
dG = (nRT/p) dp
Then integrate from p1 to p2 (image)
So G(p2) = G(p1) + nRTln(p2/p1)
Take p1 to be the standard pressure, po = 1 bar, then Go = Gibbs energy at standard pressure
So G(p) = G(po) + nRTln(p/po)
divide through by n to make into molar quantities
Gm(p) = Gom + RT ln(p/po)
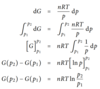
Give the expression for how Gibbs energy varies with volume, at constant temp, for an ideal gas.
G(V2) = G(V1) + nRTln(V1/V2) (const. temp, ideal gas)
Derive this expression for how Gibbs energy varies with volume, at constant temp, for an ideal gas.
G(V2) = G(V1) + nRTln(V1/V2) (const. temp, ideal gas)
- Third master eq:* dG = Vdp - SdT
- At constant temp, dT = 0, so*
dG = Vdp (const temp)
V varies with pressure p according to ideal gas eq:
pV = nRT, so V = nRT/p
Sub into eq for dG
dG = (nRT/p) dp
Then integrate from p1 to p2 (image)
So G(p2) = G(p1) + nRTln(p2/p1)
For an ideal gas, pressure is inversely proportional to volume at constant temp, so p2/p1 = v1/v2:
So G(V2) = G(V1) + nRTln(V1/V2)

Give the expression for how molar Gibbs energy varies with temperature, at constant pressure, for an ideal gas.
Gibbs-Helmholtz equation:
d/dT(G/T) = -H/T2 (const. pressure)
Derive this expression for how molar Gibbs energy varies with temperature, at constant pressure, for an ideal gas.
Gibbs-Helmholtz equation:
d/dT(G/T) = -H/T2 (const. pressure)
Find an expression for dG/dT
- Third master eq:* dG = Vdp - SdT
- At constant pressure, dp = 0, so:*
dG = -SdT (const. pressure)
dG/dT = -S (const. pressure)
Then form an equation to sub the above into
Derivative of G/T wrt T, product rule:
d/dT(G/T) = T-1(dG/dT) - T-2(G)
Do the sub
d/dT(G/T) = T-1(-S) - T-2(G)
Sub in definition of G, G = H - TS
d/dT(G/T) = T-1(-S) - T-2(H - TS)
d/dT(G/T) = -T-1(S) - T-2(H) + T-1S
d/dT(G/T) = -T-1(S) - T-2(H) + T-1S
d/dT(G/T) = -T-2H
d/dT(G/T) = -H/T2 (const. pressure)
When considering the Gibbs energies of mixtures (as necessary for discussing equilibrium), and for an ideal gas, what does the Gibbs energy of each gaseous species depend on?
Its own partial pressure, since by definition molecules in ideal gases don’t interact.
Give the definitions (verbal and eq) of partial pressure and mole fraction of gas i within a mixture.
Partial pressure, pi, in a mixture of ideal gases, is the pressure that substance i would exert if it occupied the whole volume alone.
pi = xiptot
where ptot = total pressure and xi = mole fraction of gas i
Mole fraction, xi, is the moles of i as a fraction of the total moles of all species.
xi = ni/ntot
where ni = moles of i and ni = total moles of all gases
Give the expression for how molar Gibbs energy varies with pressure, at constant temp, for an ideal gas in a mixture. Explain where it comes from.
Recall Gm(p) = Gom + RT ln(p/po) (const. temp, ideal gas)
Ideal gases don’t interact, so there is no distinction between a pure gas at pressure p, and that same gas at partial pressure pi in a mixture:
Gm,i(pi) = Gom,i + RT ln(pi/po) (const. temp, ideal gas)
where Gm,i(pi) = molar gibbs energy of gas i present at partial pressure pi, etc etc
Give the formula for the Gibbs energy of a mixture of ideal gases.
Ideal gases don’t interact, so just add the molar Gibbs energy of each species, each multiplied by its mole fraction, each at its own partial pressure:
G = nAGm,A(pA) + nBGm,B(pB) + …
then you could replace each Gibbs energy with the formula in the prev fc
Discussing the Gibbs energy of a mixture of ideal gases doesn’t apply to equilibria involving solutions, liquids or solids. So chemical potential is introduced instead.
What symbol is used for the chemical potential of say species A?
μA
Discussing the Gibbs energy of a mixture of ideal gases doesn’t apply to equilibria involving solutions, liquids or solids. So chemical potential is introduced instead.
What equation gives the Gibbs energy of a mixture, using chemical potentials?
G = nAμA + nBμB + …
Give the expression for how chemical potential of gaseous species i varies with pressure, at constant temp, in a mixture.
μ(pi) = μoi + RT ln(pi/po)
- Analagous to:*
- Gm,i(pi) = Gom,i + RT ln(pi/po) (const. temp, ideal gas)*
Give the expression for how chemical potential of ideal, dissolved species i varies with pressure, at constant temp, in a mixture.
solutions are rarely ideal, but this course assumes they are
μ(ci) = μoi + RT ln(ci/co)
where co = standard conc, 1 mol dm-3
- Analagous to:*
- Gm,i(pi) = Gom,i + RT ln(pi/po) (const. temp, ideal gas)*


