Energetics & Equilibria 4: Equilibrium Flashcards
Write the expressions for the eq constant for the following reaction involving
- gases
- dissolved species
- solids and liquids
A + B ⇔ P + Q
solids: trick question, solid species don’t contribute to expression

Why do solids and liquids in reactions not contribute a term to the eq constant expression?
Chemical potentials of solids/liquids = standard chemical potentials
I.e. µ = µo, so the term that solid A with coefficient vA = (µ/µo)vA = 1. Therefore the contribution is to multiply the overall expression by 1 – no change.
What is true of Gibbs energy at equilibrium?
∆rG = 0

Effectively, ∆rG is the slope of a graph of Gibbs energy against composition, composition being the ratio of products to reactants. See image
Give the expression linking the standard Gibbs energy of reaction and the equilibrium constant.
∆rGo = -RTlnK
Derive that ∆rGo = -RTlnK
Assume generic reaction A + B ⇔ P + Q
For a mixture of gases (NB you could do equiv calculation assuming solutions instead):
∆rG = vpμp(pP) + vQμQ(pQ) - vAμA(pA) - vBμB(pB)
Assume all species are gaseous
μ(pi) = μoi + RT ln(pi/po)
Then sub in chemical potential expressions
∆rG = vp(μoP + RT ln(pp/po)) + vQ(…) - vA(…) - vB(…)
Gather together the standard chemical potentials
∆rG = (vpμoP + vQμoQ - vAμoA - vBμoB) + vpRT ln(pp/po) + vQ(…) - vA(…) - vB(…)
Quantity in bracket = ∆rGo. Put the stoichoimetric coefficients onto the ln terms
So ∆rG = ∆rGo + RT ln(pp/po)vp + …etc
Then gather the ln terms, from here on see image

CaCO3(s) ⇔ CaO(s) + CO2(g)
Derive an expression for ΔrGo at equilibrium.
ΔrG = µ(CO2) + µ(CaO) - µ(CaCO3)
For the solids, µ = µo. For CO2 gas, write in terms of partial pressure, recalling µi(pi) = µoi + RTln(pi/po)
ΔrG = µo(CO2) + µo(CaO) - µo(CaCO3) + RTln(pCO2/po)
ΔrG = [µo(CO2) + µo(CaO) - µo(CaCO3)] + RTln(pCO2/po)
Quantity in [] = ΔrGo
ΔrG = ΔrGo + RTln(pCO2/po)
At equilibrium, ΔrG = 0, so:
ΔrGo = -RTln(pCO2,eq/po)
This demonstrates that, although solids/liquids don’t contribute to the expression for the eq constant K, they do still contribute to ΔrGo, and so they affect the value of K indirectly
- Rearrange the standard Gibbs energy equation to make K the subject
- Use this to explain how the sign of the standard Gibbs energy predicts the position of equilibrium

reaction with +ve change in standard gibbs energy proceeds to some extent. overall, going from reactants to products is associated with a rise in gibbs energy, so this isn’t spontaneous and doesn’t occur. however, it’s not a straight line from reactants to products – it’s a curve which falls then rises. at the minimum of the curve is where gibbs energy is at a minimum. this is the eq composition.
Use this graph to demonstrate the basis of Le Chatelier’s principle, e.g. why the act of removing products (altering concentration) is counteracted.
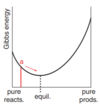
- removing product increases proportion of reactant in mixture
- graph moves from eq position to position marked a
- to return from a to eq is associated with a decrease in gibbs energy, therefore spontaneous
- so some reactants reform products to counteract the change
- Derive the temperature-dependence of the equilibrium constant
- Use this to explain why, for an endothermic reaction, increasing temperature shifts eq to products, and vice versa
Derivation
Recall ΔrGo = -RTlnK and also ΔrGo = ΔrHo - TΔrSo
So -RTlnK = ΔrHo - TΔrSo
Divide through by -RT
lnK = -(ΔrHo/R)(1/T) + (ΔrSo/R)
Interpretation
Assume ΔrHo and ΔrSo are temperature-independent over a modest temperature range.
- For an endothermic reaction, ΔrHo > 0, so -(ΔrHo/R)(1/T) is negative, so increasing T makes the term less negative, so lnK and thus K increase. Shifts eq towards products (increased K)
- For an exothermic reaction, ΔrHo rHo/R)(1/T) is positive, so increasing T makes the term less positive, so lnK and thus K decrease. Shifts eq towards reactants (decreased K)
NB this approach is only applicable for reactions which go to completion
Another method for determining the temperature-dependence of K uses the Gibbs-Helmholtz equation. Write it out
d/dT(G/T) = -H/T2 (constant pressure)
Another method for determining the temperature-dependence of K uses the Gibbs-Helmholtz equation:
d/dT(G/T) = -H/T2 (constant pressure)
Use this to derive the van’t Hoff equation:
dlnK/dT = ΔrHo/RT2
d/dT(G/T) = -H/T2 (constant pressure)
Replace with standard quantities
d/dT(ΔrGo/T) = -ΔrHo/T2 (constant pressure)
Sub in ΔrGo = -RTlnK –> ΔrGo/T = -RlnK
d/dT(-RlnK) = -ΔrHo/T2
Divide through by -R
dlnK/dT = ΔrHo/RT2
Another method for determining the temperature-dependence of K uses the Gibbs-Helmholtz equation to derive the van’t Hoff equation:
dlnK/dT = ΔrHo/RT2
Use this to derive the temperature-dependence (should get the same result as before).

For dissolved species, altering pressure doesn’t have a significant effect, but it does for gaseous species.
State how pressure affects the equilibrium constant and composition.
- For a gas phase reaction, Kp and ∆rGo are related via ∆rGo = −RT lnKp, and ∆rGo is defned under standard conditions (pressure = 1 bar), so Kp does not vary with pressure
- Composition of eq mixture changes in order to keep Kp constant
Consider the following equilibrium:
A2(g) ⇔ 2A(g)
Use the concept of the degree of dissociation to determine the pressure-dependence of the equilibrium composition.
Let α = degree of dissociation of dimer A2, i.e. the fraction which have dissociated, where α = 0 means no dissociation and α = 1 means full dissociation.
n0 moles of A2 are allowed to reach eq at pressure ptot, giving degree of dissociation α.
So neq,A2 = (1-α)no, and neq,A = 2αno, and therefore neq,total = (1+α)n0.
Partial pressure of a species i: pi = xiptot
Then write out the partial pressures of each species and derive the eq constant, then rearrange for α (image from here on).

How do biological systems drive thermodynamically unfavourable reactions?
- Couple an unfavourable reaction (ΔrGo > 0) to a favourable one (ΔrGo > 0) such that overall (ΔrGo > 0), so the reaction can occur
- Coupling occurs physically through connecting reactions closely, eg via an enzyme
- Examples:
- ATP hydrolysis is often the favourable reaction used to drive another, e.g. protein synthesis
- ATP synthesis is therefore unfavourable; it is coupled to glucose oxidation
- Photosynthetic production of glucose is not favourable, so is coupled to light energy


