Diabetes III - Obesity and Diabetes Complications Flashcards
Categories of end-organ complications in diabetes mellitus
- Glucose related
- Vascular related
- Other endocrinologic or metabolic complications
Glucose-related DM complications
- Sorbitol: In the setting of hyperglycemia, intracellular glucose is increased. Excess intracellular glucose is diverted to an alternate pathway, leading to an accumulation of sorbitol. The subsequent osmotic gradient draws water into tissue
- Advanced glycation end products: AGEs damage the basement membrane of the extracellular matrix and increase procoagulant activity, vascular permeability, and monocyte influx (a factor in development of atherosclerosis) that contribute to vascular injury
Vascular-related DM complications
Abnormalities in the endothelium and supporting cells, leading to an increase in vascular permeability.
This may be caused by local hemodynamic or inflammatory factors, such as cytokines (including IL-1), nitric oxide, growth factors (including vascular endothelial growth factor (VEGF)), and angiotensin II.
Other endocrinologic or metabolic complications
- Abnormalities in platelet function and growth factors
- Associated lipid abnormalities (high triglycerides and low HDL seen in insulin resistance)
- Hyperinsulinemia, leading to endothelial dysfunction and a prothrombotic state
Diabetic retinopathy
- May result from macular edema, hemorrhage from retinal vessels, retinal detachment, or glaucoma
- Most asymptomatic, however progression may be rapid if untreated.
- Direct relationship between degree of hyperylgycemia and risk of development
- Hyperglycemia leads to pericyte death and basement membrane thickening, resulting in increased permeability and local edema.
- Bleeding can lead to fibrosis and traction on the retina, which can cause retinal detachment.
Retinal neuropathy in type I vs type II diabetes
- Happens to 50-80% of type II, virtually all of type I
- Progression depends on duration and control of diabetes
Normal vs Diabetic retina on exam

Diabetic neuropathy may be classified into. . .
. . . Nonproliferative or proliferative.
Based on the absence of presence of neovascularization within the retina.
Diagnosing diabetic retinopathy
- Consistent history
- Dilated fundoscopic exam
- Digital stereoscopic retinal imaging
- Fluorescein angiography (to detect neovascularization)
Treating diabetic retinopathy
- Glycemic control and blood pressure control can decrease both the incidence and progression of diabetic retinopathy.
- Laser photocoagulation of new proliferative retinal blood vessels can decrease vision loss
- Intravitreal anti-VEGF injections, intravitreal steroid injections, and vitrectomy are more invasive treatments that can also be used.
Pathogenesis of diabetic nephropathy
- AGE’s can lead to irregular thickening of the glomerular basement membrane. In order to maintain filtration, pressure within the glomeruli increases, leading to enlargement of the glomeruli.
- Higher glomerular pressures lead to hyperfiltration, such that glomerular filtration rates can initially increase.
- Over time, however, increased glomerular pressures can lead to podocyte damage and development of albuminuria
Diabetic nephropathy strongly increases the risk of ___, even relative to other diabetes patients.
Diabetic nephropathy strongly increases the risk of severe cardiovascular disease, even relative to other diabetes patients.
Diagnosing diabetic nephropathy
- Urine albumin-to-creatine ratio screening: Screening tests for urine albumin should begin at 5 years after diagnosis of type 1 diabetes and at the time of diagnosis of type 2 diabetes.
- Kidney biopsy: Rarely indicated unless an additional diagnosis is possible that would require additional therapy. Changes include mesangial expansion, GBM thickening, afferent/efferent arteriosclerosis, glomerular hypertrophy, and Kimmelstiel-Wilson nodules.
Treating diabetic nephropathy
- Blood pressure control: RAAS inhibitors work both systemically and locally, and so are doubly beneficial. The risk, of course, is reduced GFR
- Glycemic control
- Smoking cessation
- Note that these can only prevent further damage, but cannot restore kidney function. Ultimately, if GFR falls low enough to be incompatible with life, dialysis or transplant are necessary.
Diabetic neuropathy mechanisms
- The metabolic mechanism, whereby elevated sorbitol in neurons exposed to hyperglycemia leads to nerve dysfunction and damage.
- The vascular mechanism, whereby vascular damage leads to ischemia which in turn causes nerve dysfunction and damage.
Symmetric sensorimotor neuropathy in diabetes
- Most common form of diabetic neuropathy
- Altered sensitivity to vibration and heat (leading to loss of sensory perception). This can manifest symptomatically as paresthesias (including numbness and/or pins and needles sensation), hyperalgesia, and allodynia.
- Longer nerve fibers are damaged first since they are the longest and most susceptible to injury (hence, neurons that innervate the toes/feet often go first).
- Decreased or absent reflexes
Autonomic neuropathy in diabetes
- Gastric/intestinal dysmotility (diarrhea or constipation, altered absorption, bloating, early satiety)
- Erectile dysfunction
- Bladder dysfunction
- Cardiac dysfunction (loss in variation in heart rate and vascular tone, resting tachycardia, postural hypotension)
- Changes in vascular tone
Acute mononeuropathies in diabetes
- Acute onset
- Involve a single nerve
- Lead to pain but with recovery typically within 6-12 weeks
Cranial and peripheral motor neuropathy in diabetes
self explanatory
Treatment of diabetic neuropathy
- Glycemic control
- Management of neuropathic pain (ex, pregabalin, a gaba analogue used in treating fibromyalgia)
Severe peripheral vascular disease in diabetes may lead to . . .
. . . gangrenous extremities and amputations
Foot ulcers in diabetes mellitus
- Loss of protective sensation in the feet (peripheral neuropathy) leading to an increase in foot trauma
- Poor blood flow (peripheral vascular disease)
- Predisposition for infections results in an increased risk for development of ulcers and subsequently amputations.
Musculoskeletal complications in diabetes mellitus
- Dupuytren’s contractures: A proliferation of connective tissue in the hand and thickening of the skin, leading to decreased mobility of the fingers.
- Charcot joint: A degenerative condition seen in weight-bearing joints characterized by bone destruction and deformity.
- Carpal tunnel syndrome
- Association between diabetes and osteoarthritis, gout, and osteoporosis, though the mechanisms remain unclear.
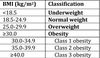
BMI
BMI = weight / height2