CI j: Nitrogen chemistry Flashcards
Name 2 things which cause N2 to form nitrous oxides in the atmosphere.
- High temperatures from combustion processes
- Lightning
N2 has a high bond enthalpy. Draw:
- A simple diagram representing its bonds
- A dot and cross diagram
- A diagram showing the types of bonds present

To represent ammonia, draw:
- A dot and cross diagram
- A full structural diagram
- Its 3D shape

Why can H+ ions be added to ammonia to form ammonium?
- Nitrogen atom in ammonia has a lone pair of electrons
- A dative covalent bond forms between the lone pair and hydrogen
To represent ammonium, draw:
- A dot and cross diagram
- A structural diagram
- How the ion is conventionally drawn
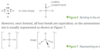
Give the name, appearance and state at room temperature of each of the following nitrogen compounds. Include details of reactionary tendencies.
- NO
- NO2
- N2O
NO = nitrogen (mon)oxide
Colourless gas (converted to NO2 in air)
NO2 = nitrogen dioxide
Brown gas (stable)
N2O = dinitrogen (mon)oxide
Colourless gas (decomposes to NO2)
Describe the acidity of the nitrous oxides.
- NO = neutral
- NO2 = acidic
- N2O = neutral
NO2 is the brown one, and the one that the other two tend to be converted to in the atmosphere. It is always the distinctive one.
Describe the water solubility of the nitrous oxides.
- NO - not very soluble (more in alcohol)
- NO2 - highly soluble
- N2O - moderate in cold water; low in hot (more in alcohol)
Write equations to show how nitrogen dioxide is formed indirectly as a result of the high temperatures of combustion processes.
N2 (g) + O2 (g) → 2NO(g)
2NO(g) + O2 (g) → 2NO2 (g)
Explain the structure of nitrogen monoxide.
•N = O
Lone pair on nitrogen, as well as an unpaired electron.
Radical.
Explain the structure of nitrogen dioxide.
O = •N = O
Trigonal planar where one of the 3 components is an unpaired electron
Angle ONO < 120o due to repulsion of electron
Radical
Why aren’t oxidation numbers typically used for naming the oxides of nitrogen?
The use of ox numbers implies an ionic nature.
Name each ion:
- NO3-
- NO2-
- Nitrate(V)
- Nitrate(III)
Write out the chemical sequence by which anaerobic bacteria reduce nitrate(V) ions. Show state symbols and oxidation states.
NO3-(aq) → NO2-(aq) → NO(g) → N2O(g) → N2 (g)
+5 → +3 → +2 → +1 → 0
Draw a dot and cross diagram and an accurate structural representation of nitrate(III).
- It’s a stabilisation of the •NO2 radical.*
- 2 “1.5” bonds rather than 1 single + 1 double.*

Draw a dot and cross diagram and an accurate structural representation of nitrate(V).

Both nitrate(III) and nitrate(V) are very soluble in water.
Aerobic bacteria in soil form nitrate(V) by nitrification, via the conversion of ammonium ions to nitrate(III).
Write equations to represent the formation of nitrate(V).
Oxidation
NH4+(aq) + 1.5O2 (g) → NO2-(aq) + 2H+(aq) + H2O(l)
NO2-(aq) + 1/2 O2 (g) → NO3-(aq)
First one can be derived via half equations:
- When ammonia is converted to nitrate(III), nitrogen’s ox state changes as follows: -3 → +3 so oxidation, producing 6 electrons
- 2 protons are required on RHS to balance charge: NH4+→ NO2- + 6e- + 2H+
- O2 (ox state 0) provides the 6e- and is converted to H2O (ox state -2), so must be 3 O atoms + therefore 1.5 O2 molecules
Describe and explain the test for nitrate(V) ions.
- Heat test solution with NaOH(aq) + Davarda’s alloy, Cu/Al/Zn
- If nitrate(V) present, ammonia gas forms:
3NO3- + 8Al + 5OH- + 18H2O → 3NH3 + 8[Al(OH)4]-
Test for ammonia by:
- Turns damp red litmus paper blue
- Forms white ammonium chloride gas when HCl(g) added (fumes from HCl(aq))
- Sharp smell
Describe and explain the test for ammonium ions.
Acid-base reaction
- Heat test solution with NaOH(aq)
- If ammonium is present, ammonia gas forms:
NH4+(aq) + OH-(aq) → NH3(g) + H2O(l)
Test for ammonia by:
- Turns damp red litmus paper blue
- Forms white ammonium chloride gas when HCl(g) added (fumes from HCl(aq))
- Sharp smell
Explain why the test below is not used if nitrate(III) ions are believed to be present. The second reaction causes a brown ring to be observed.

Nitrate(III) ions make the test unreliable by oxidising the Fe2+ ions. If nitrate(V) ions were present, the test would still be negative.
What is the balanced equation for the following reaction?
NO3- + Al + OH- + H2O → NH3 + [Al(OH)4]-
Balance what is involved in redox
N: +5 → -3 gains 8e- Al: 0 → +3 loses 3e-
LCM = 24 24/8 = 3N 24/3 = 8 Al
3NO3- + 8Al + OH- + H2O → 3NH3 + 8[Al(OH)4]-
Balance charges
3NO3- + nOH- → 8[Al(OH)4]- n = 8 - 3 = 5
3NO3- + 8Al + 5OH- + H2O → 3NH3 + 8[Al(OH)4]-
Balance the rest
As is: 14 O + 5 H → 32 O + 41 H
32 - 14 = 18 O 41 - 5 = 36 H
3NO3- + 8Al + 5OH- + 18H2O → 3NH3 + 8[Al(OH)4]-
Write a balanced half-equation for the following reaction:
NO3- → N2
+5 to 0 so reduction/gain
So add electrons + protons
2NO3- + 10e- → N2
2NO3- + 12H+ + 10e- → N2 + 6H2O
Have to add protons (rather than not, and just oxygen being formed) to balance charge.
Write a balanced half-equation for the following reaction:
NH4+ → NH3
-3 to -3 so no redox
NH4+ + OH- → NH3 + H2O
Write a balanced half-equation for the following reaction:
NO → NO2
+2 to +4 so oxidation/loss
So produce electrons + protons
NO → NO2 + 2e-
NO + H2O → NO2 + 2H+ + 2e-
Need protons on RHS to balance charge. Therefore need H2O not O2 as ox agent.


