Cell signalling: 8. Signals and receptors & 9. Transduction and response Flashcards
(31 cards)
Why is cell commmunication important?
Organisms have many different cells - communication needed to function together - emergent properties
What are the types of cell communication?
- Local signalling (cells in direct contact with each other)
- Long distance signalling (hormones / endocrine system used)
Explain local signalling in cells
- direct contact between cells - via channel proteins (gap junctions)
- close proximity - communication via chemical signalling (synapsis)

Explain long distance signalling
- Hormones and whole endocrine system used to communicate (ex for fight or flight response - adrenalin)
- Also in plants (Giberellins - for growth)

What are the types of targetting in cell signalling?
- Autocrine
- Juxtacrine (via gap junction)
- Paracrine (chemical messengers, ex synapsis)
- Endocrine (signalling via bloodstream)
Andrew Jokes Praising Elizabeth

What is the mechanism of accepting a signal in a cell?
- Reception - receptor bonds to a signalling molecule (also called ligands)
- Transduction - changing forms - message passed by series of protein changing shapes
- Response - carried by an effector
One receptor might affect many relay molecules -> many responses to one signalling molecule

What are the types of effector proteins and what are their responses?

Which molecules are used as E for cell signalling?
- ATP
- GTP - from guanisine - more commonly used in signalling than ATP - E derived by hydrolysis into GDP + iP + E - by GTPase
Structurally similar

Explain the replacement of GTP to GDP and GDP to GTP
GAP and GEF proteins aid in replacement
GTP is made not by adding a phosphate to GDP - completely new GTP is brought to the reaction

Explain protein phosphorylation
- another way for cells to communicate - activation of certain proteins by adding a phosphate group (reversible modification - activation and be deactivated) - structural conformation changes once phsophorylated
- phosphorylation performed by protein kinases (activation)
- phosphates removed by protein phosphatase (deactivation)
- most commonly phosphates added to serine, threonine, tyrosine and histidine (am a)
- very common for proteins to have multiple activation sites for phosphorylation

What are signalling cascades?
- chain signalling between different proteins - a signal is being amplified
- phosphorylation also used to amplify signals - signalling cascades (A protein phsophorylated - signals for 6 B proteins to phosphorylate - signals for 100 C proteins to phosphorylate and so on)

What are the different types of receptors?

Explain G-protein coupled receptors
- structure: form 2 parts = receptor + G protein (7 alpha helices)
- the receptor is integral transmembrane protein the - communicates outside to inside of the cell - G protein acts like a messenger between the receptor and target enzyme
- used as medication receptors
- roles: light sensitive in eyes, molecules in food as taste, scent molecules as smell, response to immune triggers, blood pressure, heart rate, digestion neurotransmitters - widely used - highly associated with disease
- when G protein bound to GTP - active state, when bound to GDP - inactive state

Explain the transmission of a signal in G-protein coupled receptors
- signalling molecule binds to the receptor - receptor is activated
- G protein interacts - lost its GDP and gains GTP - receptor acts as a GEF because aids in exchange of GDP with GTP
- activated G protein travels along membrane to activate the enzyme (common activation - phosphorylation) - conformation of the enzyme is changed - active site exposed - catalysis of reaction performed - product obtained - activation used up - enzyme inactive
- G protein detaches - its GTP converted to GDP because E given to enzyme - ready to be activated by the receptor again
- example: mechanism for adrenalin (epinephrine)

Explain tyrosin kinase receptors
- also called receptor tyrosin kinases (RTKs)
- transmembrane receptors - bind to extracellular ligand on the outside of the cell
- high numbers in eukaryotes - at leats 90 types in humans - 90 different functions
- enzyme active inside the cell - triggers internal signalling cascade - enzyme triggered kinase (phosphorylates other proteins by phosphate from ATP_)_
- ligand examples: HGF (human growth factor), NGF (nerve growth factor), EGF (epidermal growth factor), insulin
- receptor structure: two monomers - two parts: extracellular receptor binding site, intracellular downstream effect part
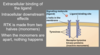
Explain RTKs mechanism of action
- RTK inactive when the two monomers are apart - active when they are combined (friendship necklace)
- when ligands bind to both receptors - move together - form a dimer (membrane fluidity allows this move to happen)
- the enzyme part of RTK activated only when all tyrosines are phsophorylated - 6 ATPs needed -> fully activated
- relay proteins approach the activated enzyme - bind to active sites - relay enzymes activated - initiate cellular signalling (chain of protein kinase activation) - cellular response (effect)
- the activated enzyme of RTK can activate different relay proteins - from the activation of one RTK - many cellular signals - many cellular responses

Explain phosphorylation cascade

What is transduction?
Transduction - change of one signal into a different type of signal (ex phosphorylation cascade)
Explain ligand-gated ion channels
- channel proteins but controlled by ions - open / closed
- used for influx of ions - cellular response
- ex synapsis - when neurotransmitter binds - ion channels open - influx of ions into postsynaptic neuron
- some channels can be opened by binding of ATP (P2X receptor family)

Which receptors are intracellular?
Steroid hormone receptors
Explain steroid hormone receptors
- some signalling molecules cross the membrane (no transport protein in the membrane) - usually lipid soluble (hydrophobic) - hormones (ex testosterone, oestrogen, cortisol) - bind to receptors inside the cell (usually very sensitive) - concentration control the size of the effect
- usually the final effect of these bindings - gene activation / inactivation - changes to mRNA - changes to proteins - hormone-receptor complexes regulate gene expression (by affecting transcription factors)
What is cross-talk?
Cross-talk - when two different signalling molecules bind to two different receptors activating two different relay proteins -> one relay protein can activate / inhibit the other relay protein (Picture 3)

Explain what are feedback loops and two examples
- Negative feedback - if exceeds conc - switches off / if conc lower - switched on
- Positive feedback - if exceeds conc - switched on - if conc lower - swicthed off
- Homeostasis - regulation of a process to keep a system stable in response to external stimuli
- Ex: lac operon (picture), p53 (the guardian of genome)

Explain the feedback loop of lac operon





