BREAST IMAGING Flashcards
Mammogram general approach
- These are screening CC and MLO views of the breasts.
- The breast tissue is comprised of
- Predominantly fatty replaced <25%
- scattered glandular tissue 25% - 50%
- Heterogenous Glandular issue 50 - 75%
- Predominately glandular/dense breasts >7%
- I am going to review the images for MicroCalficiations.
- Now I will review the images for
- Masses
- architectural distortion
- non-specific density
- focal asymmetries
- Adenopathy
- Skin thickening.
- Localisation
- Upper outer
- Upper inner
- Lower outer
- Lower inner
- Characterisation
- Previous films
- Clinical data
- I would/would not recall the patient
- Additional views
- Microcalc
- True lateral to look for teacupping. if present benign
- no teacupping: Compression magnification views.
- Tomosynthesis (CC and Lateral/MLO views)
- Are the microcalcs easily visible on USS?
- Yes -> biopsy -> Clip -> MMG to confirm position.
- Yes but too small to bx on both USS and Tomo-> early follow up in 6-12 months
- no - > easily visivible on TOMO -Yes: VACB -> clip -> MMG
- Not easily seen on USS or TOMO -> early follow up
- Microcalc
- Additional views
Architectural Distorsion
- referes to distorted breast paranchyma and appears as thin, straight lines radiating from a sing e focal point withoutan an associated discrete mass.
- Architectural distortion may be due to
- scar
- trauma
- surgery
- in the absence of trauma or surgery, architectura distrosion should always be biopsied.
Types of breast Cancers
1.
extra veiws on MMG
Cone compression view to separated overluing stucuires
Mag views uselfyl to define microcalcs
Lateral vview
Extended CC
review areas on mMg
Retromammary triangle
On the MLO view these are the central space between the best tissue and the chest wall and the lower triangle.
Thes are all typically fatty areas so the presence of a focal asymmetry in these areas
The milky Way: the retromammary fat area on the MLO
Limitations of MMG
dense breast
lobular ca - mammographically occult. Can be seen on USS.
technical limitations
current sensitivity 90%
Next step for dense breast on MMG
USS or MR
Birads
- 0 = incomplete. technically inadequate. needs follow up
- 1 = normal. no suspicious masses
- 2 = benign (fibrocystadenoma)
- 3 = equivocal/ probably benign. Call patients back for an assessment clinic. 2% chance of cancer
- 4 = suspicious. needs bx. If comes back benign, needs surgical review, hook wire localisation and WLE 2-95% cancer
- 5 = highly suspicious ie with LNs, or skin changes. If comes back benign, needs surgical review, hook wire localisation and WLE. >95%
- 6 = bx proven malignancy.
pathological lesions on MMG
- mass lesions
- arch distortion
- micro calc (casting, crushed, stone-like, powdery)
- non-specific density/asym
PASH
pseudoangiomatous stromal hyperplasia
epithelial hyperplasia
columnar cell alteration without atypia
how to bx breast lesion
16G core bx
breast MRI indications
- hight risk pt
- contralateral ca
- lobular
- ocult
- pre-op planning
- scar vs recurentce
- neoadjuvant rx
- dense, problematic mmg
- implants and ca
- carcinoma in situ
- staging
high risk group patients
- braca carriers and 1st degree relatives
- strong fhx breast ovary
- HO treated Hogkin
- lifraumeni, cowdens, lynch
- personal or current history of breast ca
- carcinoma in situ

- Mastitis
- Dr Daniel J Bell◉ and Assoc Prof Frank Gaillard◉◈ et al.
- Mastitis (rare plural: mastitides) refers to inflammation of the breast parenchyma, of which there are a number of subtypes:
- acute mastitis
- puerperal mastitis: occurs usually from infection with Staphylococcus spp. during lactation
- non-puerperal mastitis: not related to lactation, and occurs usually in older women
- plasma cell mastitis (mammary duct ectasia): uncommon subareolar inflammation without associated bacterial infection
- granulomatous mastitis: rare; usually occurs due to tuberculosis or sarcoidosis
- Clinically, the breast will be indurated, red and painful. Nipple retraction may also be evident. Nodal enlargement is common. The patient may often have systemic symptoms such as fever or leukocytosis.
- Complications
- breast abscess formation
- Radiographic features
- Mammography
- On mammography, bacterial (puerperal or non-puerperal) mastitis will usually feature ill-defined regions of increased density and skin thickening.
- Mammography
- Ultrasound
- On ultrasound, ill-defined area of altered echotexture with hyperechogenicity representing infiltrated and inflamed fat lobules, hypoechoic areas in the glandular parenchyma, and associated mild skin thickening are seen. Inflammatory axillary lymph nodes may also be encountered. Occasionally abscess formation may be visible.
- Differential diagnosis
- It is important to consider inflammatory breast cancer as a potential differential.
The affected side (left side here) show diffuse, relatively ill-defined, echogenic breast fat tissue and increased colour flow Doppler, and enlarged lymph nodes in the axilla, with no evidence of focal skin thickening or traction, well-defined mass, or nearby extra-breast tissue invasion.
The dilated lactiferous ducts are of similar appearance bilaterally, without evidence of wall thickening or internal echogenic component or debris.
Comparison of the normally appearing, non-affected (right side here) breast and axilla demonstrate the difference in echogenicity, architecture, axillary lymph nodes, (shown), and vascularity (right side not shown).
Puerperal mastitis
Puerperal mastitis
Dr Yvette Mellam and Dr Avni K P Skandhan◉ et al.
Puerperal mastitis refers to mastitis occurring during pregnancy and lactation.
On this page:
Article:
Epidemiology
Pathology
Radiographic features
Treatment and prognosis
Differential diagnosis
Related articles
References
Images:
Cases and figures
Epidemiology
It occurs most often during breast feeding and is rarely encountered during pregnancy.
Pathology
The source of infection is the nursing infants nose and throat; the organisms being Staphylococcus aureus and Streptococcus spp. Due to a breach in the nipple-areola complex, such as a cracked nipple, there is retrograde dissemination of these normal commensals. This is further favored by stasis of milk as stagnant milk is an excellent medium for bacterial growth.
Staphylococcus aureus infections tend to be more invasive and localized leading to earlier abscess formation; while Streptococcus infections tend to present as diffuse mastitis with focal abscess formation in advanced stages.
Subtypes
endemic/sporadic: majority of the cases
epidemic type: less common; can be life-threatening and is related to methicillin-resistant Staphylococcus aureus (MRSA)
Radiographic features
Mammography
not usually done
skin and trabecular thickening due to breast edema
abscess may be seen as ill-defined mass
Ultrasound
primary modality of choice
abscess: irregular, hypoechoic to anechoic mass with fluid and debris and posterior acoustic enhancement
mastitis: ill-defined, hypoechoic region
periductal inflammation
guidance for abscess drainage
Treatment and prognosis
antibiotic therapy
drainage of abscess
Differential diagnosis
Neoplasm should be suspected if the condition does not improve with antibiotic therapy.

Plasma cell mastitis
Linear, thick, ‘rod-like’ calcifications in both breasts, with a symmetrical distribution. Typical appearance of plasma cell mastitis (BI-RADS 2, benign).
Predominantly fatty breast tissue. No further findings.
Dr Edgar Lorente◉ and Radswiki◉ et al.
Plasma cell mastitis is a benign breast condition which represents calcification of inspissated secretions in or immediately adjacent to ectatic benign ducts.
On this page:
Article:
Epidemiology
Pathology
Radiographic features
Treatment and prognosis
Related articles
References
Images:
Cases and figures
Epidemiology
It is typically seen in older women (e.g. >60 years of age).
Pathology
It is thought to represent aseptic inflammation of the breast from extravasation of intraductal secretions into periductal connective tissue.
Radiographic features
Mammography
Plasma cell mastitis has a characteristic appearance. Calcifications are thick, linear, rod-like or cigar-shaped. Calcifications can be up to 10 mm long. They tend to be bilateral, often symmetrical in distribution and oriented with long axes pointing toward the nipple1. Branching may sometimes be seen.
Compared to microcalcifications of DCIS or ductal carcinoma, calcifications of plasma cell mastitis are larger in both length and caliber and have a smoother outline.
Treatment and prognosis
It is a benign entity and there is no increased risk of malignancy 3.

Mammary duct ectasia
Dr Francis Deng◉ and Dr M Venkatesh et al.
- Mammary duct ectasia is the abnormal widening of one or more breast ducts to greater than 2 mm diameter, or 3 mm at the ampulla. It can be due to benign or malignant processes.
Terminology
- Some publications use this term synonymously with periductal mastitis 7 or plasma cell mastitis 10,11, while others suggest that they are distinct entities with a different pathogenesis 8,9 .
Epidemiology
- It is more common in females in an age group of 50-60 years (i.e. postmenopausal). It is very rarely seen in males. It can occasionally be seen in children 14.
Clinical presentation
Ductal ectasia is often asymptomatic, especially when benign. However, patients with ductal ectasia may present with nonspecific breast symptoms:
- nipple discharge
- nipple retraction
- pain/tenderness
- palpable mass
Pathology
- Benign duct ectasia is characterized by chronic inflammatory and fibrotic changes. Inspissation of debris and secretions within the dilated ducts and later calcification of these ductal contents occurs. There is a known association between ductal ectasia and smoking 12.
- Intraductal malignancy can also cause duct ectasia.
Radiographic features
Mammography
- dilated linear branching densities in subareolar region
- variably present rod-like calcifications pointing towards the nipple
Ultrasound
- distended branching or tubular structures with anechoic contents measuring more than 2 mm diameter
MRI
- On T1 and T2 weighted images it appears as dilated increased signal intensity branching ducts converging towards the nipple without an overlying mass. Hyperintense signals are due to thick proteinaceous fluid or blood.
History and etymology
- It was first described by Haagensen in the year 1951 3.
Differential diagnosis
- Dilated ducts on breast imaging may be seen on many breast imaging modalities and can arise from a number of causes which can be both benign or malignant.
- physiological lactational changes
- mammary duct ectasia
- breast neoplasm 2-3
Practical points
- Bilateral, subareolar findings of duct ectasia may confidently be assessed as benign (BI-RADS 1 or 2).
- A unilateral (asymmetric) mammographic finding of duct ectasia without demonstrated stability on prior studies warrants further evaluation with ultrasound 15. Features that on ultrasound should raise suspicion for malignancy include nonsubareolar location, hypoechoic intraluminal contents, ductal wall irregularity or indistinctness, or solid parenchymal mass 9,15.
- A solitary dilated duct, a rare type of asymmetric duct ectasia, is suspicious for malignancy and biopsy should be considered (BI-RADS 4) 15.

Fat necrosis (breast)
Dr Yair Glick◉ and Dr Jeremy Jones◉ et al.
- Fat necrosis within the breast is a pathological process that occurs when there is saponification of local fat.
- It is a benign inflammatory process and is becoming increasingly common with the greater use of breast conserving surgery and mammoplasty procedures.
Epidemiology
- Most at risk are middle-aged women with pendulous breasts. The onset of fat necrosis can be considerably delayed, occurring 10 years or more after surgery 3.
Pathology
- At the microscopic level, the initial change is disruption of fat cells, with the formation of vacuoles containing the remnants of necrotic fat cells.
- The vacuoles are then surrounded by lipid-laden macrophages, multinucleated giant cells, and acute inflammatory cells.
- Fibrosis develops during the reparative phase, peripherally enclosing an area of necrotic fat and cellular debris.
- Eventually, fibrosis may replace the area of degenerated fat with a scar, or loculated and degenerated fat may persist for years within a fibrotic scar.
Etiology
- direct trauma, e.g. from a seat belt, breast biopsy, implant removal, prior reconstruction
- in everyday practice, trauma and surgery are the most common causes
- nodular panniculitis: Weber-Christian disease
- When there is calcification within the cyst wall, it is termed liponecrosis macrocystica calcificans.
Location
- There is a predilection for the subareolar and periareolar regions.
Radiographic features
Mammography
- Fat necrosis can have a very variable, sometimes alarming appearance on mammography and is often potentially confusing to the novice breast imager.
- Initially, it can be seen as an ill-defined and irregular, spiculated mass-like area.
- Associated calcification can be present, which can mimic that of more malignant entities such as DCIS.
- Note that fat necrosis of the breast can change in time with progressive calcification, so comparison with previous imaging is essential.
- Also, the changes can often be seen and correlated with the position of surgical scarring on the breast itself (refer to the technologist sheet).
- The calcification of fat necrosis is typically peripheral with a stippled curvilinear appearance creating the appearance of lucent “bubbles” in the breast parenchyma.
- Note the low-density centers.
- Tumor formation is not a part of fat necrosis although it may be clinically palpable.
- With time, it becomes more defined and well-circumscribed giving rise to an oil cyst.
- Oil cysts can have very fine curvilinear calcification of the walls.
- The center of the lesion becomes increasingly homogeneous with fat-density.
- The cyst wall calcifies in ~5%.
Breast ultrasound
- Fat necrosis may be seen as a hypoechoic mass with well-defined margins +/- mural nodule(s).
- The identification of the subtle wall nodularity in an oil cyst is a dead giveaway but takes effort and real-time imaging.
- Ultrasound of fat necrosis should always be interpreted in the context of mammographic findings.
- Aspiration of an oil cyst shows typically a milky, emulsified fat appearance.
- In the sample bottle, the fat globules can be seen drifting on the cytology before they disperse. This is the typical appearance and is immediately recognisable.
Differential diagnosis
- On ultrasound, the lesion may occasionally represent an intracystic carcinoma and mammographic correlation is recommended in these circumstances 1.
- The key to diagnosis is the history, the tech sheet and review of multiple cases.

Fibroadenoma (breast)
Dr Mohammad Osama Hussein Yonso◉ and Dr Jeremy Jones◉ et al.
Fibroadenoma is a common benign breast lesion and results from the excess proliferation of connective tissue. Fibroadenomas characteristically contain both stromal and epithelial cells.
Epidemiology
- They usually occur in women between the ages of 10 and 40 years.
- It is the most common breast mass in the adolescent and young adult population 1,3.
- Their peak incidence is between 25 and 40 years. The incidence decreases after 40 years 4.
Clinical presentation
- The typical presentation is in a woman of reproductive age with a mobile palpable breast lump.
- Due to their hormonal sensitivity, fibroadenomas commonly enlarge during pregnancy and involute at menopause.
- Hence, they rarely present after the age of 40 years.
- The lesions are well defined and well-circumscribed clinically and the overlying skin is normal.
- The lesions are not fixed to the surrounding parenchyma and slip around under the palpating fingers, hence the colloquial term a breast “mouse”.
Pathology
- A fibroadenoma is a type of adenomatous breast lesion.
- It contains epithelium and has minimal malignant potential 8.
- Multiple fibroadenomas occur in 10-15% of patients.
- Patients with multiple fibroadenomas tend to have a strong family history of these tumors.
- They are assumed to be aberrations of normal breast development (ANDI) or the product of hyperplastic processes, rather than true neoplasms.
- Fibroadenomas can be stimulated by estrogen and progesterone.
- Some fibroadenomas also have receptors and respond to growth hormone and epidermal growth factor.
- When found in an adolescent girl, the term juvenile fibroadenoma is more appropriate.
Location
- Although they can be located anywhere in the breast, there may be a predilection for the upper outer quadrant.
Associations
- cyclosporin use
- Cowden syndrome 9
Radiographic features
Mammography
- Fibroadenomas have a spectrum of features from the well-circumscribed discrete oval mass hypo- or isodense to the breast glandular tissue, to a mass with macrolobulation or partially obscured margin.
- Involuting fibroadenomas in older, typically postmenopausal patients may contain calcification, often producing the classic, coarse popcorn calcification appearance.
- In some cases the whole lesion is calcified.
- Calcification may also present as crushed stone-like microcalcification which makes differentiation from malignancy difficult.
Breast ultrasound
- Typically seen as a well-circumscribed, round to ovoid, or macrolobulated mass with generally uniform hypoechogenicity.
- Intralesional sonographically detectable calcification may be seen in ~10% of cases 2.
- Sometimes a thin echogenic rim (pseudocapsule) may be seen sonographically.
Breast MRI
- T1: typically hypointense or isointense compared with adjacent breast tissue
- T2: can be hypo- or hyperintense
- T1 C+ (Gd): can be variable but a majority will show slow initial contrast enhancement followed by a persistent delayed phase (type I enhancement curve); non-enhancing internal septations may be seen
Diagnosis
- These lesions are easily biopsied under ultrasound guidance. When a lesion has the typical features of a fibroadenoma on ultrasound and there are no clinical red flags they can be safely followed clinically.
- When lesions enlarge or have atypical imaging findings, ultrasound-guided core biopsy is a minimally invasive outpatient procedure that will give a diagnosis with virtually no complications.
- Depending on where you work, there may be a maximum diameter above which a biopsy should be done if no previous imaging is available.
- There is significant local variation in this regard.
- The reason for intervention based on size is that a phyllodes tumor may be indistinguishable from a fibroadenoma on ultrasound.
- A maximum diameter of 2.5 cm may be a useful benchmark for biopsy if you have no previous imaging. Interval enlargement is an indication for biopsy.
Treatment and prognosis
- They are benign lesions with minimal or no malignant potential. The risk of
- malignant transformation is extremely low and has been reported to range around 0.0125-0.3%.
- Indications for biopsy include:
- enlarging lesion
- atypical findings on ultrasound
- a lesion above 2.5 cm and there are no previous studies for comparison
- patient peace of mind: some patients are simply not happy with a palpable mass in the breast without a histological diagnosis; this is a valid and reasonable indication for biopsy

Complex fibroadenoma
Complex fibroadenoma
Dr Daniel J Bell◉ and Radswiki◉ et al.
Complex fibroadenoma is a sub type of fibroadenoma harboring one or more of the following features:
epithelial calcifications
papillary apocrine metaplasia
sclerosing adenosis and
cysts larger than 3 mm
Epidemiology
Complex fibroadenomas tend to occur in older patients (median age, 47 years) compared with simple fibroadenomas (median age, 28.5 years).
Pathology
They fall under the broad group of adenomatous breast lesions. Complex fibroadenomas are often smaller than simple fibroadenomas (1.3 cm compared with 2.5 cm in simple fibroadenomas). When histopathology on core biopsy reveals a higher-risk lesion, such as atypical lobular hyperplasia, excisional biopsy may be indicated to rule out malignancy.
The clinical relevance is not clear.
Radiographic features
There are no clear cut mammographic or sonographic features that distinguish complex from simple fibroadenomas.
Complications
There are numerous reports that the general risk of developing cancer in the breast parenchyma is elevated among women with complex fibroadenomas; these women are 3.1-3.7 times more likely to develop breast cancer than women in the general population (compared with a relative risk of 1.9 times in women with non-complex fibroadenomas). ~50% of these tend to be lobular carcinoma in situ (LCIS), ~20% infiltrating lobular carcinoma, ~20% ductal carcinoma in situ (DCIS), and the remaining 10% are infiltrating ductal carcinoma .

Lymphocytic mastitis
Dr Henry Knipe◉◈ et al.
Lymphocytic mastitis, also known as lymphocytic mastopathy or sclerosing lymphocytic lobulitis, is a rare benign inflammatory disease of the breast that can mimic breast cancer.
Terminology
Diabetic mastopathy is a closely-related entity although it is sometimes used synonymously in the literature.
Clinical presentation
Lymphocytic mastitis may present as a palpable mass, which may be painful and may be bilateral.
Pathology
Lymphocytic mastitis is associated with autoimmune disease (e.g. Hashimoto thyroiditis, systemic lupus erythematosus, Sjogren syndrome) 1.
Macroscopic appearance
Dense fibrous tissue with hard lesions, that can be large (up to 6 cm) 4.
Case:
Ultrasound revealed an irregular hypoechoic lesion of 3 cm, with marked posterior acoustic shadowing (the lesion was considered as BI-RADS 5).
Echo color Doppler shows a peripheral straight vessel penetrating the lesion, but no internal vessels.
STAT DX:
Terminology
Diabetic fibrous breast disease (DFBD), diabetic fibrous mastopathy, lymphocytic mastopathy, sclerosing lymphocytic lobulitis
Chronic inflammation & stromal changes in women with diabetes
Imaging
Usually subareolar or central breast
Mammography: Noncalcified dense asymmetry
US: Hypoechoic region/mass with indistinct margins, marked posterior shadowing without internal flow
MR: T2 hypointense with focal or regional gradual heterogeneous enhancement
Biopsy for definitive diagnosis: Lesions typically harder than invasive carcinomas; target edge of lesion
Top Differential Diagnoses
Invasive carcinoma (ductal, lobular), lymphoma
Stromal fibrosis of breast
PASH
Desmoid tumor/fibromatosis
Pathology
Prominent myofibroblasts, perivascular lymphocytic infiltrates surrounding lobules, ducts, blood vessels
Dense collagenous stroma; may be due to abnormal glucose deposition on collagen
Clinical Issues
Hard, palpable, mobile, nontender masses; may be multiple, bilateral; premenopausal woman with longstanding insulin-dependent diabetes, some with autoimmune thyroid disease; 20-year average interval between diabetes onset and mass
No increased risk for development of invasive cancer
Self-limited course; may recur
TERMINOLOGY
Synonyms
Lymphocytic mastopathy
Diabetic fibrous breast disease (DFBD)
Diabetic fibrous mastopathy
Sclerosing lymphocytic lobulitis
Definitions
Periductal, perilobular, and perivascular lymphocytic infiltrate associated with dense interlobular stroma
Occurs primarily in women with longstanding insulin-dependent (type 1) diabetes
IMAGING
General Features
Best diagnostic clue
Hypoechoic, avascular shadowing mass with indistinct margins
Location
Usually subareolar or central breast
Mammographic Findings
Noncalcified dense asymmetry
Usually dense breast parenchyma: Often occult
Ultrasonographic Findings
Hypoechoic region or mass with indistinct or spiculated margins and marked posterior shadowing
Typically avascular on color/power Doppler
Stiff on elastography
MR Findings
T2 hypointense when densely fibrotic
Focal area or regional slow gradual heterogeneous enhancement; may not enhance
Image-Guided Biopsy
Lesions typically denser than invasive carcinomas
14-g core devices may not penetrate easily or may not adequately sample lesion for diagnosis: Consider VAB
Target edge of lesion
Imaging Recommendations
Protocol advice
Ultrasound most useful imaging tool
Suspect in patients with longstanding type 1 diabetes
Biopsy required for definitive diagnosis
DIFFERENTIAL DIAGNOSIS
Invasive Carcinoma (Ductal, Lobular)
Hypoechoic mass; indistinct margins, Ca⁺⁺ common in IDC
Stromal Fibrosis
Focal asymmetry; hypoechoic shadowing mass
Asymmetric Breast Tissue
Stable for years; interspersed fat
Pseudoangiomatous Stromal Hyperplasia
Solid, round or oval mass; hypoechoic ± cysts
Benign myofibroblastic, hyperplastic process
Lymphoma
Masses of malignant cells; stromal proliferation not prominent; markedly hypoechoic and hypervascular
PATHOLOGY
General Features
Lesions composed of primarily fibrotic and inflammatory cells with stromal, keloid-like fibrosis
Stromal changes may be due to abnormal glucose deposition on collagen
Gross Pathologic & Surgical Features
2-10 cm in size
Cut surface often indistinguishable from surrounding breast parenchyma
Microscopic Features
Prominent myofibroblasts and dense perivascular lymphocytic infiltrates surround lobules, ducts, and blood vessels; lymphocytes predominantly B cells
Distinguish from lymphocytic lobulitis associated with carcinomas, which are predominantly T cells
Basement membranes of ducts and lobules may be markedly thickened; lobules small in size, sparse in number
Dense, paucicellular collagenous stroma; stromal cells positive for fibroblast and myofibroblast markers (CD34, smooth muscle actin, desmin, CD10)

FIBROCYSTIC CHANGE
Stat Dx
Terminology
- Fibrocystic change (FCC): Spectrum of histopathologic changes, including cysts, fibrosis, apocrine metaplasia (AM), Ca⁺⁺, inflammation, epithelial hyperplasia, and sclerosing adenosis (SA)
Imaging
- Mammography: Dense, fibroglandular tissue; fluctuating pattern of cysts; scattered diffuse, randomly grouped punctate, amorphous Ca⁺⁺, milk of calcium
- US: Cysts, complicated cysts, clustered microcysts, complex cystic and solid mass (uncommon); heterogeneous echotexture; occasional Ca⁺⁺, hypoechoic mass (fibrosis)
- MR: Cysts, rim-enhancing inflamed cysts, scattered foci, focal or regional nonmass enhancement
Top Differential Diagnoses
- Atypical ductal hyperplasia (ADH), lobular neoplasia
- DCIS
Pathology
- Nonproliferative findings: Adenosis, cysts, inflammation due to leakage of cyst contents, AM, fibrosis
- Proliferative findings: SA, usual ductal hyperplasia (UDH)
Clinical Issues
- Pain frequent, often cyclical, usually in outer breasts
- More common in premenopausal women
- Palpable cysts; greenish or black nipple discharge
- Dense breasts and masses: ↓ mammographic sensitivity
- Frequent false-positives on all breast imaging modalities
- Proliferative changes (UDH and SA) carry slight (~ 1.5-2.0x) ↑ risk of developing breast cancer in either breast
Diagnostic Checklist
- Range of imaging findings concordant → ensure adequate sampling
Ultrasonographic Findings
- Simple cysts; complicated cysts with debris; clustered microcysts (benign findings)
- Complex cystic and solid masses: Thick wall ± thick septations
- Mixture of fibrosis and cystic changes
- Ruptured or inflamed cysts
- Often require biopsy
- Tissue distortion with central cysts and intervening echogenic fibrosis
- Discrete masses due to dense fibrosis
- Mixed echogenicity mass: Fibrosis usually echogenic
- Irregular, hypoechoic with shadowing, often requiring biopsy
- Scattered echogenic foci due to Ca⁺⁺
- Occasionally in cysts (i.e., milk of calcium)
- Color or power Doppler: Internal flow excludes cyst
- Elastography: Fibrosis can be stiff; bull’s-eye artifact with cysts on some strain elastography
DDx
- Lobular Neoplasia
- Atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS) typically occult on mammography
- Adjacent to or (rarely) contain amorphous Ca⁺⁺
- Can cause indistinct, irregular masses on US, MR
- Atypical Ductal Hyperplasia
- 29% of grouped amorphous Ca⁺⁺ are high-risk lesions: ADH, ALH, LCIS, flat epithelial atypia (FEA)
- Ductal Carcinoma In Situ
- Grouped pleomorphic, fine linear, amorphous Ca⁺⁺
- Ca⁺⁺ in linear or segmental distribution favor DCIS
- Clumped linear or segmental NME on MR
- Invasive Ductal or Lobular Carcinoma
- Most often irregular, spiculated mass
- Architectural distortion
- Invasive ductal carcinoma can show associated Ca⁺⁺
PATHOLOGY
Etiology
- Thought to be related to excess hormonal stimulation &/or hypersensitivity of breast tissue
- Common in premenopausal women
- FCC ↓ in women on tamoxifen
- Associated abnormalities
- Papilloma, radial sclerosing lesion
Demographics
Age
More common in premenopausal women; ↓ in postmenopausal women
Epidemiology
- 81% of reduction mammoplasty specimens have FCC
- Higher socioeconomic status, fewer pregnancies, less lactation than controls
- Trend to ↑ with nulliparity, later menopause
- ↑ cyst formation in postmenopausal women on hormone replacement, especially estrogen alone
- Natural History & Prognosis
- Nonproliferative FCC: No increased risk of carcinoma
- Proliferative FCC: ↑ relative risk of developing carcinoma in either breast 1.5-2.0x
Consider
- FCC represents spectrum of normal breast physiology
- Not indication for diagnostic mammography in absence of focal symptoms or abnormalities on screening
- Focal pain not sign of malignancy, though US can be performed
- Ca⁺⁺ are frequent manifestation of FCC
- Low threshold for magnification CC, 90° views to fully characterize Ca⁺⁺
- Ensure adequate sampling of suspicious Ca⁺⁺
- Common source of false-positives leading to biopsies
- Result of FCC on core biopsy may be discordant
- Concordant: Coarse heterogeneous Ca⁺⁺; hypoechoic, shadowing mass
- Distortion should be explained by SA or radial scar, otherwise discordant
- MR-guided biopsy yielding fibrosis or FCC → ensure adequate sampling: Up to 10% may have missed lesion
- Image Interpretation Pearls
- Diffuse, scattered, bilateral similar findings favor benign
- True lateral mammogram helpful to confirm benign milk of Ca⁺⁺
Epidemiology
- Very common entity (observed clinically in up to 50% and histologically in 90% of women 6).
- Fibrocystic change is unusual before adolescence.
- These are most often diagnosed between the ages of 20 and 40 with the peak before or at menopause.
- As compared to the general population, women with nonproliferative lesions have no significant elevation in risk of developing a breast carcinoma, while those with the proliferative disease have a greater risk.
Radiographic features
Mammography
- breasts show heterogeneous and usually dense parenchyma
- partially circumscribed masses may be present reflecting cysts
- tea-cup, low-density round calcifications in multiple lobes
Ultrasound
On ultrasound, findings may show:
- prominent fibroglandular tissue in the area of a palpable nodule; however, no discernible mass
- small cysts in the mammary zone

Intraductal papilloma of breast
Dr Yuranga Weerakkody◉ and Radswiki◉ et al.
- Intraductal papillomas are the most common masses within the milk ducts of the breast.
- They are benign tumors but may contain areas of atypia or carcinoma.
- The most common symptom is nipple discharge.
Epidemiology
sex
- almost exclusively in women
- extremely rare in males 9
age
- classically most common during 40-50 years of age (average 48 years old)
- increasing use of breast ultrasound has resulted in more frequent detection of papillary lesions in younger, asymptomatic women
Clinical presentation
- often asymptomatic (incidental imaging finding)
- nipple discharge: especially if unilateral, spontaneous, persistent
- bloody or clear (serosanguineous) nipple discharge, often less than six months duration
- more common in central versus peripheral papillomas 10
- bloody nipple discharge is thought to be due to twisting of the papilloma on its fibrovascular pedicle, leading to necrosis, ischemia, and intraductal bleeding
- bloody nipple discharge may have higher association with atypical or malignant lesions 11
Pathology
- Papillomas are proliferative tumors originating from the walls of milk ducts, typically growing within the duct and tending to cause local ductal obstruction.
- They are composed of monotonous epithelial/myoepithelial cells encompassing a papillary fibrovascular core, and characteristically grow to form smooth well-circumscribed nodules.
- Papillomas are typically small (<10 mm) lesions but may range from 3 mm to >2 cm 10.
- They most commonly occur ~3.5 cm from the nipple but may occur anywhere from anterior to posterior depth 10,11.
- Increasingly, the central question in the assessment of breast papilloma is whether there is any evidence of cellular atypia.
- Any finding suggestive of more than merely benign proliferation are generally grounds for surgical excision of the entire lesion.
- In addition, papillomas have been reported occurring adjacent to other significant lesions such as atypical ductal hyperplasia or DCIS.
- Papillomas may be solitary or multiple.
- Multiple papillomas, especially more than 5 lesions, are considered papillomatosis.
- There may be a higher rate of associated malignancy with multiple papillomas.
Subtypes
sclerosing papilloma of the breast
Location
- They may be classified by location:
- central: within a major subareolar duct, often solitary
- peripheral: occur within the terminal duct lobular unit, may be multiple
Radiographic features
Mammography
- Mammograms are frequently normal (particularly with small intraductal papillomas).
- When imaging findings are present, they include solitary or multiple dilated ducts, a circumscribed benign-appearing mass (often subareolar in location), or a cluster of calcifications.
Galactography
- Galactography usually reveals a filling defect or other ductal abnormalities, such as ectasia (usually between the nipple and filling defect), obstruction, or irregularity.
- However, these findings are non-specific.
- Galactography may outline the number, location, extent, and distance from the nipple.
Breast ultrasound
- Papilloma may be seen as a well-defined solid nodule or intraductal mass which may either fill a duct or be partially outlined by fluid - either within a duct or by forming a cyst.
- Color Doppler will demonstrate a vascular stalk.
- A dilated duct can be frequently visible sonographically.
MRI
- Most commonly appear as modestly-T2-bright, circumscribed, solid enhancing lesions. Morphological characteristics on MR may be quite variable:
- shape: can be oval/round (~75%) or irregular (~25%) shape 10,11
- margin: smooth or irregular (spiculation suggests malignancy) 10
- consistency: mostly solid (~90%), but can be cystic or complex cystic 11
- Signal characteristics
- T1: isointense to slightly hypointense relative to breast glandular tissue 10
- T2: hyperintense to breast glandular tissue, but less bright than cysts 10
- T1 C+ 10rapid early enhancement
- absolute enhancement rate may be somewhat less than DCIS
- may be a homogeneous or heterogeneous pattern
- may show peripheral (“rim-like”) hyperenhancement on delayed images
- dynamic enhancement pattern non-specific; all 3 types of kinetics (persistent, plateau, and washout) have been described 10,11
- DWI/ADC: restrict diffusion (high DWI, low ADC values) 10
Nuclear Medicine
PET-CT
- may be associated with mildly increased (less than liver) FDG-avidity
- at least one report of markedly increased (SUV 10-12) avidity in a papilloma with strong expression of GLUT-1transporter 8
Treatment and prognosis
- Most centers treat solitary intraductal papillomas with surgical excision, even after benign biopsy, to exclude components of atypia or neoplasia. However, there is some controversy surrounding this, with some groups suggesting that clinical follow-up is sufficient if there is no atypia (including ADH) on core biopsy 7.
- Given the increased risk of malignancy over a woman’s lifetime when this lesion is diagnosed, compliance with screening recommendations for such patients is strongly advisable.
- According to a consensus committee of the College of American Pathologists, women with this lesion have a relative risk of 1.5-2 times for developing invasive breast carcinoma in their lifetime.
Differential diagnosis
The differential includes other solid tumors that can occur in the large ducts, specifically:
- ductal carcinoma in situ
- invasive ductal carcinoma with an in situ component
- papillary carcinoma of the breast can mimic an intraductal papilloma (particularly on ultrasound)
For ultrasound appearances also consider:
- inspissated secretions within a dilated duct may mimic papillomas but have no associated vascularity
- complex breast abscess with debris: solid component mobile
- fat necrosis: also no Doppler vascularity
USS Case: Ultrasound demonstrates a solid, vascular mass within a cystic space, with clearly visualised intraductal extension. Appearance in keeping with a papillary lesion.

what is usual ductal hyperplasia?
“Usual hyperplasia” means there is excessive growth of benign cells in an area of the breast, but the cells don’t look abnormal. This can happen along the inner lining of the breast duct (tube that carries milk to the nipple) or the lobule (small round sac that produces milk).
https://www.pathologyoutlines.com/topic/breastepithelialductalhyperplasia.html
Definition / general
Benign intraductal proliferation of progenitor epithelial cells with varying degrees of solid or fenestrated growth
Essential features
Component of fibrocystic changes
Mild cytologic variability
Streaming growth pattern with fenestrated spaces and lack of cellular polarity
Immunoreactive for high molecular weight cytokeratins
Associated with slight increase in subsequent breast cancer risk (1.5 - 2 times)
Terminology
Also called epithelial hyperplasia, intraductal hyperplasia, hyperplasia of usual type, ductal hyperplasia without atypia, epitheliosis
Epidemiology
Mean age is 54 (N Engl J Med 2005;353:229)
Most significant finding in 20% of benign breast biopsies (Cancer 2006;106:732)
Sites
Terminal duct lobular units
Occasionally, extralobular ducts
Etiology
No specific etiologic factors
Clinical features
No specific clinical findings
Diagnosis
Diagnosis by histologic examination of tissue removed via biopsy or surgical excision
Radiology description
No specific mammographic findings; occasional examples are associated with microcalcifications
Can involve an underlying lesion (e.g. radial scar or papilloma) that is identified on imaging
May show enhancement on magnetic resonance imaging (Arch Pathol Lab Med 2017;141:1513)
Prognostic factors
Associated with 1.5 - 2 times increased risk for subsequent breast cancer (N Engl J Med 2005;353:229, Cancer 2006;107:1240)
Risk may be slightly higher for patients with a positive family history of breast cancer (Cancer 2006;107:1240)
Indicator of general breast cancer risk rather than direct precursor lesion

Sclerosing adenosis of the breast
Dr Francis Deng◉ and Radswiki◉ et al.
- Sclerosing adenosis (SA) is a benign proliferative condition of the terminal duct lobular units characterized by an increased number of acini and their glands.
- It manifests as multiple small, firm, tender nodules, fibrous tissue, and variable microcysts within the breast.
- It is sometimes placed under the category of borderline breast disease.
Clinical presentation
- Many women with sclerosing adenosis experience recurring pain that tends to be linked to the menstrual cycle.
- In most cases, sclerosing adenosis is detected during routine mammograms or following breast surgery.
- A biopsy is required to confirm the diagnosis, because the condition may be difficult to distinguish from breast cancer by imaging.
- Sclerosing adenosis can appear as a focal or diffuse lesion.
- It is not physically palpable in 80% of the cases, although in some cases may cause skin retraction.
Pathology
- Sclerosing adenosis is a type of adenosis in which enlarged acini become slightly distorted by surrounded stromal fibrosis (“sclerosis”). The normal lobular architecture of the breast is maintained but becomes exaggerated and distorted.
Associations
- Sclerosing adenosis can be seen as a component of other proliferative lesions:
- intraductal and/or sclerosing papilloma
- complex sclerosing lesion
- fibroadenoma
- breast cancer, both invasive and in situ
Radiographic features
Mammography
- Sclerosing adenosis has a wide range of mammographic presentations, and can be difficult to distinguish from an infiltrating carcinoma:
- mass, with irregular to well-defined contours
- architectural distortion
- microcalcifications
- present in 40-55% of cases 5,6
- may be amorphous, pleomorphic, or punctate 3,5
- more commonly clustered, although may present in diffuse scattered distribution 3,5
Treatment and prognosis
- Although not considered a pre-malignant lesion, sclerosing adenosis is considered an independent risk factor for the development of subsequent breast cancer 3,5.
- Studies suggest that women with sclerosing adenosis may have approximately 1.5-2 times as high a risk of developing breast cancer.
MMG:Large area of parenchymal distortion with a small number of concerning microcalcifications. Note that the centre of the lesion has a relatively low density.
USS: This is the ultrasound images of the lesion above. The microcalcifications are visible on ultrasound. The lesion is relatively speaking noted to have a low echogenicity centre, i.e. there is no central mass lesion.


Radial scar
Dr Alexandra Stanislavsky◉ and Dr Jeremy Jones◉ et al.
Radial scar, or complex sclerosing lesion, is a rosette-like proliferative breast lesion. It is not related to surgical scarring. Some authors, however, reserve the latter term to lesions over 1 cm 5.
It is an idiopathic process with sclerosing ductal hyperplasia.
Its significance is that it is a mimicker of scirrhous breast carcinoma. Although some classical differential descriptions exist (see below), these cannot be relied on, and the diagnosis must not be made on radiological features alone. Furthermore, there is an association with atypical ductal hyperplasia and carcinoma.
Epidemiology
The reported prevalence of radial scars is 0.1-2.0 per 1,000 screening mammograms. Radial scar is very rare in women younger than 40 years and older than 60 years. Most often in women between 41-60 years 12-13 .
Clinical presentation
They are usually not palpable. Clinical examination of the breast containing regions of radial scar is often normal, although in about 25% of cases radial scars can be palpable. They do not cause skin thickening or retraction. Lesions are usually small and detected by mammography when they are at least 5 mm in size. Lesions <1 cm are called radial scars, while larger ones are often referred to as complex or radial sclerosing lesions.
Pathology
A radial scar is a benign hyperplastic proliferative disease of the breast. Proposed possible causes include localized inflammatory reaction and chronic ischemia with subsequent slow infarction.
Histopathologically radial scars contain hyperplastic tissue cells and a central fibrous core, with radial extension of tubular structures (the spiculated peripheral borders), mimicking infiltrating carcinoma. This tubular formation has two rows of cells, epithelial and myoepithelial 9-10. The malignant potential is two times greater than in the normal population without radial scar 11-12.
Associations
In approximately 30% of cases, a radial scar is associated with ductal carcinoma in situ and tubular carcinoma of the breast. The occurrence of these is higher when there is associated atypia on histology.
Other associations include 4:
atypical ductal hyperplasia
atypical lobular hyperplasia
Radiographic features
Mammography
A radial scar has a spiculated appearance similar to carcinoma, but the center tends to be a translucent, low-density area rather than a mass. The breast tissue behind the lesion is almost visible through the lesion.
The relatively low density of the center is a relevant and visible difference between carcinoma and a radial scar.
A carcinoma tends to have a dense center.
With radial scars there is no dense center; in fact, the lesion is usually as dense centrally as peripherally.
There is no “attempt” at forming a mass in a radial scar.
The spicules running from the center are in general longer and gracile than those of a carcinoma (look at the image in Case 1 and 2 thoughtfully. These are representative images).
The spicules are described as long and thin with radiating radiolucent linear structures, which against a radiolucent fat background gives a black star or dark star appearance 6.
Microcalcifications are possible but rare in a radial scar.
However, unlike a carcinoma, features such as skin thickening and retraction are characteristically absent 2.
There is no visible scirrhous reaction in the radial scar.
Its mammographic appearance is also similar to a post-surgical breast scar and can vary markedly with differing projections (i.e. CC vs MLO).
Ultrasound
On ultrasound, a radial scar is often ill-defined and disturbs the architecture of surrounding breast parenchyma. The lesion is usually round, oval or lobulated. Variable internal echoes can be found. Some radial scars show retro-acoustic attenuation.
MRI
Features are replicated as described in the aforementioned modalities. There will be spiculation and archiectural distortion. Non-enhancement of the lesion favors a benign process. Enhancement suggests an underlying malignancy.
Treatment and prognosis
A radial scar is considered a high-risk breast lesion and histological differentiation from associated carcinoma is required. FNA and core biopsies can underestimate the underlying associated malignancy and are controversial. The lesions are biopsied and removed.
Differential diagnosis
Differential considerations for mammographic appearances include:
breast cancer: a central mass tends to form. The spicules are shorter and thicker and there is retraction of the parenchyma; however sometimes the invasive lobular carcinoma, due to lack of the E-cadherin and diffuse infiltration of the tumor cells, it can be impossible to distinguish it from radial scar
post-surgical breast scar: in practice this is rarely if ever a source of confusion; it is really rare to find post-surgical scarring with such long spicules as a radial scar and you also have the history on the technologist notes and if all else fails, the scar on the patient’s skin
MMGThe image shows a well defined density upper left which was a cyst. The spiculated parenchymal distortion centrally was the abnormality being worked up. Note the relatively low density centre and the long spiculated bands running concentrically from the centre.
MMG 2
Stromal distortion in the right central breast with a central lucency. No mass.

Atypical ductal hyperplasia
Atypical ductal hyperplasia
Dr Yuranga Weerakkody◉ and Radswiki◉ et al.
Atypical ductal hyperplasia (ADH) is a histologically borderline lesion that has some, but not all the features of ductal carcinoma in situ (DCIS). Sometimes the distinction between ADH and DCIS is simply on the basis of the number of ducts involved.
Pathology
Atypical ductal hyperplasia is a lesion with a lot of malignant potential. It lacks the strict criteria for ductal carcinoma in situ. A lesion which is qualitatively similar to DCIS yet quantitatively is inadequate (< 2 ducts involved) is termed as atypical ductal hyperplasia 3.
Treatment and prognosis
ADH is considered a high risk breast lesion. Therefore surgical excision is advised as under-estimation of ductal carcinoma in situ is encountered when atypical ductal hyperplasia is retrieved on a large core needle biopsy (up to one-third of cases may be upstaged to DCIS). Tamoxifen may be used as a chemopreventative agent.
Atypical lobular hyperplasia
associations
Increased Risk? how much?
Atypical lobular hyperplasia
Dr Daniel J Bell◉ and Radswiki◉ et al.
Atypical lobular hyperplasia (ALH) is a pre-malignant lesion of the breast which falls at the milder end of the spectrum of lobular neoplasia. It is therefore considered a part of borderline breast disease.
Clinical presentation
It is usually asymptomatic and mammographically occult and is invariably found incidentally, in a biopsy specimen obtained for another lesion.
Pathology
ALH represents a proliferation of monomorphic cells which are morphologically identical to lobular carcinoma in situ (LCIS). The distinction is that ALH occurs in a non-distended lobule or small lobular duct, whereas LCIS is characterized by distention.
Not surprisingly, there is a spectrum of change from ALH to LCIS. To reflect this spectrum and to avoid the connotation of malignancy per se, use of the term “lobular neoplasia” has been advocated by some authors.
Treatment and prognosis
Treatment is controversial. Some centers surgical excise while others do not.
Compared with the general population, the risk of subsequent breast cancer is 4-6x higher after a diagnosis of ALH (compared to approximately 11x after a diagnosis of LCIS).
Subtypes of Ductal carcinoma in situ
which is more aggressive?
what type of calcificaitons
Ductal carcinoma in situ
Ductal carcinoma in situ (DCIS) refers to a breast carcinoma limited to the ducts with no extension beyond the basement membrane, as a result of which the disease has not infiltrated the parenchyma of the breast and the lymphatics and cannot therefore metastasize.
Epidemiology
The detection of ductal carcinoma in situ has increased markedly in recent years secondary to the widespread use of screening mammography, and it now accounts for 25-40% of mammographically detected breast cancers 1,3. It also accounts for approximately 15-20% of all detected breast cancers.
Associations
up to 11% of predetermined ductal carcinoma in situ on imaging may have an invasive component at the time a biopsy is done 2
20-25% of DCIS revealed on core biopsy may have invasive ductal carcinoma following surgical excision
Risk factors
Risk factors for ductal carcinoma in situ are similar to those for invasive carcinoma and include:
increasing age
family history of breast cancer
nulliparity
age of 30 years or older at the birth of the first child
Clinical presentation
Although most patients are asymptomatic, some present with nipple-related disease (nipple discharge or Paget disease of the breast) or have palpable abnormalities.
Pathology
Ductal carcinoma in situ (DCIS) is the non-obligate precursor of invasive breast carcinoma. In the context of “overdiagnosis” the low grade DCIS cases found on screening mammography are likely to cause the number of cases where the diagnosis of breast malignancy has been made but could conceivably not have been fatal to the patient. Remember that to try and guess the grade of DCIS on the mammogram images is not plausible or reproducible. Low grade DCIS is not a dangerous disease and there is actually some thought on following the disease with MRI after a histological diagnosis has been made.
Ductal carcinoma in situ is not a single entity, but rather a spectrum of disease 3. In essence, it refers to breast epithelial cells that have become “cancerous” but still reside in their normal place in the ducts and lobules 10.
Markers
In some situations, immunohistochemical staining for E-cadherin may help to differentiate from lobular carcinoma in situ.
Subtypes
The traditional classification broadly divided ductal carcinoma in situ lesions into two types mainly based on central necrosis, grade, and cell type:
comedo - large cell: more aggressive form; also referred to as comedocarcinoma
non-comedo - small cell: less aggressive; can be further divided into
cribriform
micropapillary
papillary
solid
A new pathological classification of DCIS is based on nuclear atypia and degree of necrosis.
Radiographic features
Mammography
There are varied mammographic manifestations of ductal carcinoma in situ, with casting-type calcifications being the most common (present in 50-75% of cases 3). Other manifestations include a soft-tissue opacity either with or without associated calcifications.
Although DCIS calcifications may assume varied appearances, linear calcifications are more likely to be associated with comedo-type DCIS, while granular calcifications are more often correlated with non-comedo DCIS.
Occasionally DCIS appears as a simple mass or asymmetry without calcification (~8% of cases) 12.
There may be a significant discrepancy between the distribution of the disease as seen on the mammogram and the distribution of the disease on the pathology specimen of the breast as examined by the pathologist. In general, the calcification underestimates the distribution of DCIS in the breast, i.e. not all the DCIS calcifies.
Breast ultrasound
One of the benefits of identifying a corresponding sonographic abnormality in women with mammographically detected DCIS is to use ultrasound to guide interventional (e.g. biopsy/hook wire) procedures. A microlobulated mild hypoechoic mass with ductal extension and normal acoustic transmission is considered the most common feature in sonographically detected DCIS.
With good quality ultrasound and enough effort, it is possible in everyday practice to identify the DCIS process as it grows in the ductal system of the breast. It is quite possible to identify those minute wild and crazy calcifications of DCIS in the ducts itself and ultrasound-guided biopsy of DCIS is now an everyday procedure.
Breast MRI
DCIS most commonly appears as non-mass enhancement, most commonly with a segmental or linear distribution and clumped or heterogeneous internal enhancement pattern 14. Within non-mass enhancement, the clustered ring internal enhancement pattern is most specific for malignancy, usually DCIS 15.
Treatment and prognosis
Treatment options for ductal carcinoma in situ include mastectomy, lumpectomy with breast irradiation, or, for patients with small lesions (<1-2 cm) of low-grade DCIS, lumpectomy alone.
This disease is likely the precursor of invasive ductal carcinoma at a stage when the therapy is potentially curable. The advantage of diagnosis of DCIS is that the chances of encountering metastatic disease are in theory zero when compared to invasive ductal carcinoma. With the introduction of screening mammography, the decreased mortality of breast carcinoma is in some part due to the identification of DCIS and effective therapy. In large screening programmes, DCIS makes up to 30% of malignancies diagnosed.
Breast asymmetry
thi sis common and normal as long as there are n o other findings
Mor MCQ, an asymmetric breasrt should make you think about the ‘SHRINKING BREAST” of INVASIVE lobular br. ca..
Is nipple enhancemnet on Contrast MRI normal?
Yes. dont call it pagets.
where do most breast cancers start?
TDLU
Terminal duct lobular unit
- lactiferous sinus
- major duct
- lobules
Should you worry about calcs which appear to follow ducts?
What are these Calcs called?
yes. should worry about
linear or segmental.
Where is the majority of the blood flow to the breast from?
Lymph drainage?
- 60% via the internal mammary.
- the rest is via the lateral thoracic and intercostal perforators
- 97% of lymph drains to the axilla.
- 3% to the internal mammary nodes
axillary node levels
- the axilla is subdivided into 3 separate levels using the pec minor
- supposedly drainage is in a step wise fashion from Lv 1 to 2 to 3 and finally to the thorax
What are rotter nodes
- nodes between the pec minor and major.
- fancy name = high yeild
- same as level 2

AUNT MINNIE
- non funcitonal muscle next to the sternum that can simulate a mass.
- about 5% of ppl have one and its usually unilateral.
- Should only be seen on CC. Never on MLO
Sternalis muscle
Assoc Prof Craig Hacking◉◈ and Radswiki◉ et al.
The sternalis muscle is an uncommon anatomic variant of the chest wall musculature and is of uncertain aetiology and function. Its importance lies in that it should not be mistaken for a pathological lesion.
Epidemiology
Cadaveric studies have shown that the muscle is present in ~5% (range 1-8%) of both males and females and is twice as often unilateral as bilateral.
Gross anatomy
The sternalis muscle runs from the jugular notch of the manubrium superiorly, to approximately the caudal (inferior) aspect of the sternum. It is found adjacent to the medial edge of pectoralis major.
Radiographic features
Mammography
The mammographic appearance of the sternalis muscle is variable. Typically it is visible in the medial aspect of the breast on the craniocaudal mammogram and appears as a small soft tissue density/mass abutting the chest wall. Its margins and shape are variable (can range from flame shaped to an irregularly rounded density) 3. The muscle is usually not seen on the standard MLO or ML views. It typically measures 1-2 cm in maximum dimension.
As long as the radiologist is aware of the entity, there is usually little confusion. Ultrasound or CT or even MRI may be obtained for confirmation when the diagnosis is uncertain.
The sternalis muscle should be considered in the differential diagnosis if a posteromedial mass is noted on the CC view 2.
CT
appears as a flat parasternal muscle, longitudinal in orientation 6
Additional work up
In occasional situations, a “cleavage/valley view” may help to confirm bilaterality.

what is a milk streak
what is its significance
Effects of estrogen and progesteron on breast bud development
Should you bx a breast bud?
- the MILK STREAK is the embryologic buzz word to explain the location of the normal breast and location of ectopic breast tissue
- The most common location for ectopic bresat tissue is in the axilla
- The second most comon is the inframammary fold
- Extra nipples are most commonly in the same locations but can be anywhere along the milk streak.
- at birth, both males and females can have breast enlargement and produce milk from maternal hormones.
- As girls enter puberty, their ducts elongate and branch (estrogen)
- then their lobules proliferate (progesterone effects).
- If you bx a breast bud you could damge it and potentially damage breast development.
Changes of the breast thru the menstural cycle,
- Menstural
- follicular phase
- d 7 - 14
- Estrogen dominates
- best time to have both MMG and MRI
- Luteal Phase
- d 15 - 30
- Progesterone dominates
- breast tenderness
- breast density increases slightly
*
- follicular phase
Breast changes with HRT
HRT
- breasts get more dense
- especiialy estrogen-progesterone combos
- breast pain can occur
- typically peaking n the first year
- Fibroadenomas - who like to draink estrogen can grow
Galactocele
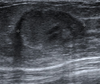
- benign fat containing lesions
- Br2
- typically seen on cessation of lactation.
- usually subareolar
- variable appearance but can have an AUNT MINNIE look with a fat fluid level
- Can become an abscess.
Galactocele
Dr Adam Eid Ramsey and Radswiki◉ et al.
Galactoceles, also referred as lactoceles, are the most common benign breast lesion typically occurring in young lactating women; however, they mostly happen on cessation of lactation 1.
Clinical presentation
Patients usually present with a painless breast lump occurring over weeks to months. The lesion can present as single or multiple nodules and can be unilateral or bilateral.
Complications
Secondary infection with development of a breast abscess.
Pathology
It is essentially a retention cyst resulting from lactiferous duct occlusion. Diagnosis can be achieved with percutaneous aspiration 8. Biochemical analysis of material aspirated from galactoceles shows a variety of proportions of proteins, fat, and lactose. Macroscopically, the milk within the galactocele may appear white and of usual viscosity if fresh, or thickened if the liquid is older.
Location
There may be predilection towards the sub-areolar region.
Radiographic features
Mammography
Mammographic appearance of galactocele can be varied depending on the fat and protein content and the consistency of the fluid. Based on these, the galactoceles could appear like a:
pseudolipoma: due to significant fat content, the mass seems radiolucent
fat-fluid level within cyst: when fat and water are present, and the milk is in a fresh liquid state, a characteristic fat-fluid level is seen due to viscosity difference. This can be demonstrated on mediolateral view with the beam horizontal to the upright patient
pseudohamartoma: seen when contents are old milk and water. Due to highly viscous old milk, significant separation of fat and water is not possible and hence gives a hamartoma-like appearance on the mammogram
Ultrasound
Ultrasound appearances can be widely variable. Sonographic characteristics according to one study is as follows 3:
cystic/multicystic: ~50%
mixed (cystic + solid): ~37%
solid: ~13%
Colour Doppler interrogation will show a lack of blood flow.
In uncertain cases, an aspiration is recommended in the first instance which will classically yield milky fluid 10.
Treatment and prognosis
They are benign lesions and spontaneous resolution occurs in a vast majority of cases. A small percentage have a residual collection that may mimic a fibroadenoma or complex cyst.
History and etymology
The term galactocele derives from the Greek words galatea meaning milky white and -cele (-coele) meaning pouch 9.
Differential diagnosis
General considerations on imaging include:
lactating adenoma: seen as a solid lesion and may show colour flow within it
breast abscess: has a different clinical presentation, but can develop as a complication
fibroadenoma: classically ovoid or almond shaped with smooth border, internal homogeneous echogenicity and acoustic enhancement 7
carcinoma of the breast: especially for certain lesions on ultrasound

breast changes in pregnancy
pregnancy
- tubes and ducts proliferate
- the breast gets a lot denser and more hypoechoic on USS.
- USS may be your best bet iff you have a mass
Lactation
- you can bx a breast that is getting ready to lactate/lactating. You just need to know there is the risk of creating a milk fistula
- if you make one, they will have to stop breast feeing to stop the fistula.
- it can get infected - uncommon.
Breast changes in perimenopause and menopause
Perimenopausal
- shortening of the follicular phase means the breast gets more mprogesterone exposure.
- most progesteron exposure means more breast pain, more FCC and more breast cyst formation
Menopause
- the floppy stage
- lobules go down
- ducts stay but may become ectatic
- fibroadenomas will degen. they like estrogen
- they will get their pop corn calc
- secretory salcs will develop 15-20 years after menopause
Pituitary prolactinoma and effects on breasts
or medications (antipsychotics) can create bilateral increased density of the breasts.

Breast within a breast
Hamartoma
lactating adenoma
- Looks like a fibroadenoma
- may actually be a chraged up fibroadenoma they like to drinj estrogen
- usually they are multiple
- if you get [presson follow up reommendation for these i would say 4-6months post partum, post delivery or after cessation of laction via uss.
- they usually radpidly regress after you stop lactation.
When do you get a LMO view?
The MLO is the standar, but somttimes you need a LMO.
The answer is women with kyphosis or pectus excavatum.
or to avoid a medial pace maker/central line
Localising a lesion
with CC, MLO and Lateral
- Start with MLO and CC.
- if you can see a medial lesion in the CC, and cant see/localise it on the MLO, then get a lateral and it should rise ie Muffins rise *medial rises on the Lateral.
- If you see a lateral lesion on the CC, and cant localise it on the MLO, it will ‘sink’ on the lateral. Lead/lateral sinks.
*
Breast MRI indications
13
- Huigh risk screening
- Personal Hx
- Family hx
- High risk lesions
- ADH
- ALH
- LCIS
- mantle radiotherapy
- Li-Fraumeni syndrome
- Brain cancers
- Breast
- Leukaemias
- Sarcomas
- Adrenocorticocarinomas
- Cowden
- Multiple hamatoma syndrome
- breast cancer
- thyroid cancver
- dysplastic cerebellar gangliocytoma, occurs when in association with Lhermitte-Duclos disease (LDD)
- mucocutaneous lesions: present in >90% of cases
- trichilemmomasmucocutaneous papillomatous papules
- Multiple hamatoma syndrome
- Bannayan-Riley-Ruvalcaba
- hamatomatous syndrome
- Extent of disease eval in contralat and ipislat breast
- positive marges
- To asses residual disease post treatment
- Axillary adenopathy unknow primary
- Posterior lesion to assess chest wall invasion
Architectural Distorsion
- referes to distorted breast paranchyma and appears as thin, straight lines radiating from a sing e focal point withoutan an associated discrete mass.
- Architectural distortion may be due to
- scar
- trauma
- surgery
- in the absence of trauma or surgery, architectura distrosion should always be biopsied.


