The Endocrine System Flashcards
(245 cards)
HORMONES RELEASED BY THE ENDOCRINE SYSTEM ARE RELEASED INTO THE BLOOD.
TRUE OR FALSE?
True
WHAT IS NEGATIVE FEEDBACK?
Once set point is reached, production/release of hormones are stopped
CLASSES OF HORMONES
1. Amines/Amino acids- tyrosine
Thyroid hormones; bind to thyroid receptors (nuclear receptors= regulate gene transcription)
Adrenaline/noradrenaline= bind to G-protein coupled receptors to bring about intracellular signaling via cyclic AMP
2. Peptides/proteins
E.G insulin, made up of 135 amino acids, works by binding to the receptor tyrosine kinase, which in turn activates intracellular signaling via a phosphorylation cascade to bring about its effects.
3. Steroid hormones
Sex hormones, e.g. Oestrogen
Glucocorticoids
These all work by activating _ receptors which effectively work as transcription factors, regulating gene transcription
Nuclear
WHAT ARE THE THREE CLASSES OF HORMONE?
Amines/Amino Acids
Peptides/Proteins
Steroid Hormones
HOW DOES ADRENALINE BRING ABOUT ITS EFFECT?
Binds to G-protein coupled receptors to bring about intracellular signaling via cyclic AMP
THE ENDOCRINE SYSTEM
Hypothalamus and pituitary release hormones that control thyroid, adrenal and gonads.
Heart releases ANP which is a hormone involved in _ balance.
Thymosin’s function in the _ system.
Melatonin is involved in regulating sleep and waking cycles.

Sodium
Immune
ENDOCRINE GLANDS
oMajor morhphological feature is that glands are ductless (cf. salivary glands)
oRichly vascularized (good _ supply).
oSecrete messengers directly into circulation
oMay be primary glands (e.g. pituitary, thyroid, adrenals)
oOther organs may have secondary endocrine function (e.g. brain (hypothalamus), heart, kidney, GI tract)

Blood
CELL-TO-CELL SIGNALLING
Intracrine= producing products that signal within cell
Autocrine= release products that act back on itself
Paracrine= Release things that affect neighbouring cells
Endocrine= products are secreted into the blood stream and can travel a distance to their target cells
Neuroendocrine= modified nerve cells that can secrete hormones into circulation directly.

ENDOCRINE FUNCTIONS
Endocrine organs release hormones that are important in four broad areas:
oReproduction
o Growth and development
o Maintenance of internal environment
o Regulation of energy
ENDOCRINE ORGANS RELEASE HORMONES THAT ARE IMPORTANT IN WHICH FOUR AREAS?
oReproduction
o Growth and development
o Maintenance of internal environment
o Regulation of energy
HORMONES
oProduced by _ and released directly into circulation
oPresent in low concentrations (10-7 - 10-12 M)
oBind to specific, high affinity recognition sites or receptors on/in target cells
oSingle hormone may have different tissue-specific effects
oSingle function may be regulated by different hormones
Glands
AMINE HORMONES
oCatecholamines derived from tyrosine
•adrenaline, noradrenaline
oThyroid Hormones also derived from tyrosine
•thyroxine, triiodothyronine
oIndoleamines derived from tryptophan
•Melatonin
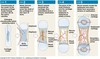
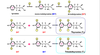
ADRENAL CATECHOLAMINE SYNTHESIS

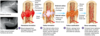
THYROID HORMONE SYNTHESIS
Thyroid hormones synthesised from tyrosine and iodine (iodine is essential).
T4- relates to the number of iodine residues.
In adrenal gland= converted to adrenaline and noradrenaline
Thyroid gland- iodinated in a cell specific pathway

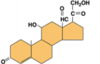
STEROID HORMONES
Coloured bits= tetra planar ring structure= common to all of these molecules as they are all synthesised from the same precursor molecule (cholesterol)
Steroids= lipophilic so they can enter and leave cells easily, but need to be transported in the blood stream bound to other proteins as they are lipophilic

STEROID HORMONE SYNTHESIS
- Starts with a hormone binding (for example) to a G-protein couple receptor.
- Causes adenyl cyclase to produce cyclic AMP
- Cyclic AMP phosphorylates protein kinase A
- This causes PKA to phosphorylate other proteins (cholesterol esterase in this case)
- CE enters cells in the form of LDL (low density lipoprotein)
- Cholesterol esterase frees the cholesterol from the protein- cholesterol then transported into mitochondria and the enzymes required for steroid hormone synthesis are located here.
- Some modifications go on in the SER
- Steroid hormone produced and released into cytoplasm, can then diffuse straight out of cell into circulation (due to being lipophilic)

DESCRIBE THE PROCESS OF SYNTHESISING A STEROID HORMONE
- Starts with a hormone binding (for example) to a G-protein couple receptor.
- Causes adenyl cyclase to produce cyclic AMP
- Cyclic AMP phosphorylates protein kinase A
- This causes PKA to phosphorylate other proteins (cholesterol esterase in this case)
- CE enters cells in the form of LDL (low density lipoprotein)
- Cholesterol esterase frees the cholesterol from the protein- cholesterol then transported into mitochondria and the enzymes required for steroid hormone synthesis are located here.
- Some modifications go on in the SER
- Steroid hormone produced and released into cytoplasm, can then diffuse straight out of cell into circulation (due to being lipophilic)
PEPTIDE AND PROTEIN HORMONES
PEPTIDES
Short amino acid chains e.g.
- ADH (9 AA)
- Oxytocin (9 AA)
Polypeptides e.g.
- Insulin (135 AA)
- Prolactin (198 AA)
Proteins
Thyroid stimulating hormone
Follicle stimulating hormone
Growth hormone
PEPTIDE AND PROTEIN HORMONES: SYNTHESIS
Release by exocytosis as prohormone or hormone into blood stream.
Proteins and peptides are packaged/stored in secretory vesicles- capable of being released straight away on demand.

HORMONE RECEPTORS
The ability of a cell to respond to a hormone depends upon the presence of receptors for that hormone on or in the target cell.
The number of receptors for a hormone can increase (up-regulation) or decrease (down-regulation).
May be:
oCell surface receptors
oIntracellular receptors
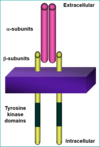
CELL-SURFACE RECEPTORS

Cell surface receptors- G-protein coupled receptors mostly
Tyrosine kinase receptors- binding of ligand causes a phosphorylation of the receptor intracellularly that recruits a signalling cascade that brings about the cellular response
INTRACELLULAR RECEPTORS

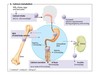
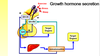
HORMONE RELEASE
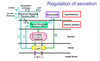
Hypothalamus and pituitary regulates- regulatory hormone released from hypothalamic neurone in response to stimulus, which acts on the endocrine cells in the anterior pituitary and causes hormone 1 to be released, which reaches the target endocrine organ and causes a second hormone to be released, gets into circulation and to the target cells= response

ENDOCRINE COMMUNICATION
- Messages disseminated from glands to effector via circulation
- Relatively slow transfer of information
- Can be long lasting
- All cells contacted, specificity conferred by receptors
- Slow maintenance of cellular homeostasis