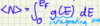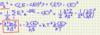Stats Flashcards
(81 cards)
State principle of equal a priori probabilities
At equilibrium, an isolated system can be found in any of its microstates with equal probability.
What is the macrostate?
A collection of microstates with a common property; same value of that property (combinations of boxes with a common property).
Define temperature as a partial derivative
Use fundamental relation of thermodynamics (1st law with chemical potential).
What is meant by microstate?
A description of a system where all values of the dynamical variables are exactly specified
Why fluctuations in system properties are rarely observed in macroscopic systems?
- standard deviation about modal macrostate goes like 1/Sqrt(N)
- so as N-> infinity, standard dev. ->0
- hence system rarely strays from the modal macrostate
What is meant by an irreversible process?
- System and surroundings cannot be returned to original conditions when process is reversed
- due to WD against dissipative forces
- Entropy is a state function, so the system entropy change is zero, although not of the surroundings (not of the surroundings what?
Boltzmann entropy of a system
- S = klnΩ
- Ω = number of microstates of a macrostate (i.e. microstate multiplicity)
Gibbs entropy
- Pi is prob. of occupation of a macrostate
- generalisation of Boltzmann entropy
- Boltzmann only applies to isolated systems where energy is conserved
- e.g. microcanonical ensemble
- or the total sum of canonical & grand canonical ensembles i.e. the reservoir
- Gibbs -> Boltzmann
- pi = 1/Ω for all in i
- hence SB = klnΩ
- pi = 1/Ω for all in i
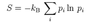
What does the Fermi-Dirac function represent?
- describes a distribution of particles over energy states in systems consisting of many identical particles
- particles obey the Pauli exclusion principle (half integer spin in thermodynamics eqm)

Conditions between system and environment that allow us to employ a microcanonical ensemble
- System is isolated with environment
- hence system cannot exchange energy or particles with its environment
- energy of system remains exactly known as time goes on
Conditions between system and environment that allow us to employ a grand canonical ensemble
- System can exchange energy particles
- hence states of system can differ in both total energy E & total number of particles N
What is a degenerate Fermi gas?
- Single particle states are occupied with probability 1 up to max. single particle energy (EF = Fermi energy)
- whilst all single particle states with E > EF are empty
- All energy levels from 0 to EF are fully occupied
- occurs at T=0 & approximately kT << EF
What happens when the temperature is much less than the Fermi temperature?
- Degenerate Fermi gas is formed when T << TF (TF = fermi temp. =EF/k)
- and also formed at absolute zero.
State the canonical partition function
- s = index for microstates of the system
- β = 1/kT
- T = temp. of reservoir
- Es = total energy of system in the corresponding microstate

What is the canonical partition function of N indistinguishable particles given single particle state Z1?
- ZN = Z1N/N!
- is the exception for identical particles
- generally the parition fn of an entire system is the product of the individual parition fns
- so if subsystems have same physical properties then
- ZN = Z1N <=> lnZN = NlnZ1
- non-interacting & distinguishable
- more generally
- Z = product of ZJ <=> lnZ = sum of ln ZJ
- for Debye model, the sum with ln ZJ is integral over frequencies
- so if subsystems have same physical properties then
What is meant by thermal equilibrium?
- 2 systems are in thermal equilibrium if they are in thermal contact
- i.e. have the same temperature.
What is the meaning of extenstivity? Which state variables in the expression for Gibbs free energy G = E + pV -TS are extensive?
- Extensive variable depend on N
- hence is proportional to the amount of the substance
- State functions are extensive
- so E, V and S are extensive
- T, p, μ are intensive
- since they stay constant with changing N
What is dG? And hence show that G = μN
- Gibbs free energy is the “free” energy available in a system to do useful work
- related to chem. pot. μ=dG/dN
- if μ is non-zero,
- particles will enter or leave system
- so G changes
- this can be shown by using 1st law at const. T and p.
What is meant by a particle bath?
- a surrounding reservoir in which the chemical potential is constant
- so there maybe exchange of particles in the system with the surroundings (reservoir)
Give an example of physical property of a degenerate fermi gas that is different from classical expectation.
.
What is the microstate multiplicity if Q indistinguishable objects are shared between N distinguishable boxes? What is the multiplicity if the boxes are indistinguishable?
- number of ways of placing Q indistinguishable items in N distinguishable boxes
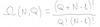
Why is it important to take into account particle indistinguishability when modelling stats therm properties of a system?
- All permutations of the particles give the same state of the system
- so leads to over-counting and degeneracy**
- for correct sum over the states, must divide by N!
- If it is a lattice => indistinguishable
- distinguishability is revelant in counting configuration
State the grand canonical partition function
- i = index of microstate
- Ni = total particle number in the ith microstate
- Ei = total enegy in the ith microstate
- μ = chemical potential of reservoir
- T = temp of reservoir
- related to Grand potential (Φ = -kTlnZG)
- whereas canonical parititon is related to F
- F = -kTlnZ
- whereas canonical parititon is related to F

What is the grand canonical partition function for fermions?
- no more than 1 fermion can occupy the same single-particle state
- N0 = 0 for E = 0
- N1 = 1 for E = ε
- so summation series ends at 1
- Z = 1 + e-(E-μ)/kT