Atomic Flashcards
What is meant by reduced mass?
- effective mass of a system once centre of mass motion is separated
- 1 particle orbiting the common centre of mass

What is meant by configuration, term and level? Give an example of each
- Configuration
- arrangement of the occupancy of number of e-s for a given orbital
- L
- Carbon: 1s2 2s2 2p2
- arrangement of the occupancy of number of e-s for a given orbital
- Term
- for a given configuration, arrangement of number of e-s are split into terms to include L and S for e-s outside closed shells & subshells
- L & S
- 1D
- for a given configuration, arrangement of number of e-s are split into terms to include L and S for e-s outside closed shells & subshells
- Level
- for a given term, arrangement of e-s are split into levels to include J which arises from spin-orbit interaction
- energy splitting from LS coupling
- levels are eigenstates of J2, Jz, L2, S2
- J, L & S
- 5D2
State Hund’s 3 rules
Lowest energy when
- For a given configuration, term with max. multiplicity 2S+1
- bigger S => lower E
- For a given multiplicity, term with largest L
- bigger L => lower E
- For a given term
- outer shell less than half-full
- lower J => lower E
- outer shell more than half-full
- bigger J => lower E
- half full
- no multiplet splitting (thought it was the lower J….?)
- outer shell less than half-full
What are the dipole allowed transitions for many electron atoms?
Consider rigorous and approximate selection rules.
Rigorous rules: (I changed te order so they’re now in order of strength)
- ΔJ = 0, ± 1 {J=0 => J’=0 Forbidden}
- ΔMj = 0, ± 1 (no effect unless there is a B or E field)
- Laporte rule - parity must change
Approximate rules:
- ΔS = 0
- ΔL = 0, ± 1 {L = 0 => L’ = 0 Forbidden} (Δl = ± 1 for 1 e-)
Strong transitions:
- atomic transitions are stronger the more dipole selection rules they fulfil
Dipole selection rule for Hydrogen.
Laporte rule - parity switch
Δl = ± 1
Δm = 0, ± 1
Δs = 0
If LS coupling is significant:
Δj = 0, ± 1 (but no j = 0 => j’ = 0)
List the approximate order of strength of the dipole transitions, including those that are forbidden.
- ΔS =/= 0 - Weakly forbidden (stronger transition than ΔL =/= 0, ±1)
- ΔL =/= 0, ±1 - Forbidden (stronger than J and Laporte rule transition)
- Laporte rule violation - strongly forbidden (weaker transition)
- J = 0 => J’ = 0 - Most strongly forbidden (weakest transition)
What is metastability?
- spontantenous decay from excited state i to all final states f is a forbidden dipole transition i.e. Aif ≈ 0
- any transition from i this unlikely to occur
- e- in metastable state i is trapped there
What do the quantum numbers l, s, j, ml, ms, mj represent and what values do they take?
These are the angular momentum quantum numbers.
- l = Orbital ang. mom; l = 0,1,2,…,n-1
- gives magnitude of orb. ang. mom => l(l+1)ħ2
- ml = orbital. mag. mom; -l,…,l
- gives component of orb. ang. mom => mlħ
- s = spin ang. mom; s = 1/2 (fermions), 0 (bosons)
- gives magnitude of spin ang. mom => s(s+1)ħ2
- ms = spin mag. mom; -s,…,s
- gives component of spin ang. mom => msħ
- j = total ang. mom; j = | l - s |,…, | l + s |
- gives total ang. mom. => j(j+1)ħ2
- mj = total mag. mom; -j,…,j
- gives component of total ang. mom => mjħ
- mj = total mag. mom; -j,…,j
- gives total ang. mom. => j(j+1)ħ2
Sketching the first few spherical harmonics.

Which quantum numbers are needed to completely specify the electrons state in hydrogen?
n, l and m
How do n, l and m arise from geometrical constraints on the wavefunction.
- n - arises from the need for the radial solution to converge
- => leads to energy quantisation, n is an integer.
- l - need for polar solution to converge, l is an integer.
- m - need for φ(…) = φ(…+2π), m must be an integer.
How do we find the radial probability density (measure of finding e- at distance from the nucleus)?
Give the number of peaks for the curve of a particular (n,l) state.
- total probability density for the wavefunction is split into radial and angular parts - don’t forget the spherical coordinates (Jacobian gives r2)
- radial probability density = integral of |Rnl(r)2| r2 from 0 to infinity
- spherical harmonics are normalised
- radial probability density = integral of |Rnl(r)2| r2 from 0 to infinity
- Can sketch the distribution for different quantum states in Hydrogen
- (n - l) peaks

Using the hydrogen radial distribution curves, what is meant by “electron penetration”” and what are the consequences for the order in which orbitals are filled?
- Electron penetration describes degree to which e-s in diff. (n,l) states penetrate region occupied by 1s electrons
- varies with l (and n, but less so)
- The more they penetrate this region, the less shielding and the more effective nuclear charge they feel.
- hence more tighly bound by the nucleus
- => have lower energy levels
- Since orbitals are filled in order of increasing energy, states with lower l are filled first
- For a given n, probability of finding e- near nucleus decreases with increasing l (further from nucleus) since centrifugal barrier pushes e- out so radius of sphere is greater
What is the Zeeman effect?
- splitting of spectral lines caused by energy shifts (arise from LS coupling) associated with magnetic dipole moment interacting with an external magnetic field is applied
Describe the weak field Zeeman effect.
- Normal zeeman:
- Atoms have zero net spin: closed shells/subshells
- Consider effect of only orbital angular momentum - μL
- Splitting only from interaction of L with B
- Anomalous zeeman:
- Net spin is non-zero
- Splitting from both L and S interaction with field
- => ΔE = μBBgJMJ (energy shift between the level splittings)
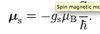
Describe the Paschen-Back case.
- B field is strong enough to uncouple L and S which then precess independently about the direction of the B field.
- Energy splitting now just the sum of the splitting due to L and S independently
- Can determine if field is strong by comparing it to LS splitting - if zeeman splitting is > the smallest LS splitting => strong field.
- ΔEL = μBBgLML
- gL = 1
- ΔES = μBBgSMS
- gS = 2
- ΔEL = μBBgLML
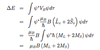
What is the Stark effect?
- Splitting of energy levels due to the application of an external E field.
What is the Quadratic Stark effect?
- atoms with no intrinsic dipole moment
- field polarises e- density inducing dipole moment p
- which is proportional to Eext
- so interaction energy VE = -p.Eext = -(1/2)αE2ext
- α = dipole polarisability
- hence varies quadratically with field
- interaction of dipole moment with E field => energy splitting
- energy shift given by eqn
- where A & B are level dependent constants
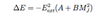
What is the linear stark effect?
- Atoms with intrinsic dipole moment (e.g. excited states of H & H-like atoms which have l-degeneracy)
- E field causes mixing of states with m =/=0
- applied E field in z direction connects states with Δl = ±1 & Δml = 0
- due to electric dipole selection rules
- applied E field in z direction connects states with Δl = ±1 & Δml = 0
- States with m=0 are centre on x-y plane but E in z direction
- E field pulls e-s to left or right and causes a mixing eigenstates of s and p states which then experience a +ve or -ve energy shift of ΔE
- Perturbing potential does not act on states where e- distributions are concentrated in x-y plane
- eigenstates of H = H0 + eEextz are also eigenstates of H0 so no work in mixing wavefns => energy is linear in Eext
- exctied state behaves as if it had electric dipole of magnetic 3ea0

Describe the Stern-Gerlach experiment
- demonstrated the quantisation of e- spin in 2 orientations
- silver atoms beam in the s state placed in non-uniform B field
- atoms experience a force along B field direction
- Since mag. moment of atoms can classically point in any direction => spread expected?
- 2 beams in opp directions observed
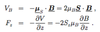
Linear stark energy diagram
- ψ+ ( sum of states) has e- density shifted towards -z, so mag. dipole is direction of E => ΔE < 0
- ψ- (diff. of states) has e- density shifted towards +z, so dipole antiparallel to E => ΔE > 0
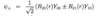
What is the quantum defect and how does it vary with the quantum numbers n and l?
- Quantifies departure from Hydrogenic behaviour - correction term to n.
- Alkali atom has single optically active (valence) electron
- further out it is (higher l), the smaller the effective nuclear charge it feels (due to inner e-s & their shielding effects)
- and the closer to a hydrogenic e- it is
- further out it is (higher l), the smaller the effective nuclear charge it feels (due to inner e-s & their shielding effects)
- Quantum defect decreases with l and n, although more slowly with n.
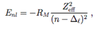
What is jj coupling?
- high Z atoms where spin-orbit interactions >> e-e interaction ( spin-spin or orbit-orbit interactions)
- individual orbital angular momenum combines with corresponding individual e- spin to from individual total angular momentum
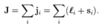
How to calculate the quantum defect
- Note in the original quantum defect formula, the energy represents the energy above the ground state
- => this is equivalent to the energy of the particular (n,l) state subtracting the ionisation energy (ground state energy)
- Replace the E in the formula with Enl with (Enl - Eion)
- Eobserved = Eion + Enl(outer electron)
- => Eobs - Eion/hc = -RMZeff2/neff2
- Zeff = 1 for alkali metal atoms

What is L-S coupling (Russell-Saunders coupling)?
- low Z atoms when e-e interaction dominates
- individual e- spin combines to form S
- individual orbital angular momenta combines to form L
- L & S couple to form J
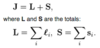
How to calculate the limit when an external B field considered to be strong?
- When spin orbit splitting ≈ separation of the sub-levels due to weak B field
- ΔESO ≈ gJμBB
What is meant by hyperfine structure?
- energy level splitting from coupling of net nuclear spin I and J (F = I + J)
- Inclusion of net nuclear spin causes further energy perturbation besides spin-orbit coupling
- interaction of net nuclear spin I with atom’s internal magnetic field
- Inclusion of net nuclear spin causes further energy perturbation besides spin-orbit coupling
What is Pauli exclusion principle for multielectron atoms? And what does this imply about their wavefunctions? Why can’t electrons have the same quantum numbers?
- e-s are fermions so each e- in multi e- atom must be described by a different spin-orbital
- total wavefns must be antisymmetric wrt exchanged of e-s
- since no 2 fermions can occupy the same quantum state
- if nlm = n’l’m’ then it would not satisfy the antisymmetry of the total wavefn
What are the 3 processes that occur when a 2 level system is in eqm with a radiation field?
- Absorption
- atom absorbs photon from field & is excited to higher E state
- rate of absorption is proportional to pop. of ground state (N) & energy density U
- atom absorbs photon from field & is excited to higher E state
- Spontaneous emission
- atom decays from excited state emitting a photon
- rate of decay is proportional to excited state pop. (N2)
- atom decays from excited state emitting a photon
- Stimulated emission
- photon from field causes excited atom to decay and emit photon
- rate of emission proportional to excited state pop. N2 & energy level U
- photon from field causes excited atom to decay and emit photon
How can alkali atoms be cooled to sufficiently low temps to form Bose-Einstein condensate?
- To achieve cooling, atoms must be sufficiently slowed to about 1cms-1
- (at room temp. atoms have velocities of several hundred ms-1)
- atoms are bombarded with many photons with energies close to transition frequency
- many since one has too little effect as pphoton << patoms
- atoms subjected to pair of counterpropagating (photon) laser beams
- Atoms see photons as doppler shifted
- beam towards (opposite direction to) atom will be blueshifted (higher frequency)
- beam in (same) direction of atoms motion will be redshifted (lower frequency)
- probability of scattering (absorption + re-emitting) a photon depends exponentially on how close a laser is tuned to resonance
- blueshifted [v’= vL(1-v/c)] => closer to resonance so probability increases (mainly absorbed)
- redshifted [v’ = vL(1-v/c)] => further from resonance so probability decreases
- Upon scattering, net momentum of p = hvL/c transferred to atom
- atom decays and emits photon causing atom to recoil
- Direction of emission random so effect of recoil averages to 0 => net momentum transfer to atoms against atoms direction of motion
- Frictional force on atoms depends on velocity (viscous damping)
- faster atoms experience larger force slowing them down
- => narrowing of momentum distribution as T decreased to around 10-6 K
- atom trap wtih 3 lasers along each x, y, z axis, and inhomogenous B field added to confine atoms
- BEC (all atoms occupy & is described by same wavefn) formed at around 10-7 ~ 10-9 => requires further techniqe of evaporative cooling (letting “hotter” atoms escape)
What is Moseley’s law? And when does the law apply?
- vif = frequency of main x-ray emission line
- Z = atomic number
- S = screening constant (depends on series)
- Zeff = Z -S
- SK = 1
- SL = 7.4
- Cn = empirical constant independent of Z
- This law is used to find the frequencies of characteristic x-rays => applied in characteristic x-ray spectra
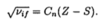
What are the K-series, L-series and M-series? And what are the subscripts within each series?
Transitions form series (groups of lines) which are defined by final state of transitions nf
- nf = 1 => K-series
- nf = 2 => L-series
- nf = 3 => M-series
Within each series, Δn = ni - nf is denoted by subscripts
- Δn = 1 => α
- Δn = 2 => β
- Δn = 3 => γ
What are the selection rules for vibration-rotation transitions for (ground electronic state of a heteronuclear) diatomic molecule? What frequency range do these transitions occur?
- Δv = ±1 (vibrational - from selection rules of harmonic oscillator)
- |J - J’| = 1 (rotational)
- Infra red frequencies
What is the Franck-Condon principle? How does it account for relative intensities of various vibrational bands in transitions between different molecular electronic states? What is the Frank-Condon factor in terms of initial and final state wavefns?
- distribution of final vibrational states after transitions between molecular eelctronic states are determined by overlap of vibrational wavefns between ground and excited state (vibrational energy levels)
- transitions between vibrational states typically occur for states in which overlap is greater since transition is much faster than nuclei speed, therefore nuclei don’t move far in this time
- hence smaller difference in nuclear coordinates of these states
- so states with bigger overlaps have higher transmission probability
- => higher intensity bands
- I = Franck-Condon Factor
- v” = vibrational wavefn of lower state (final)
- v’ = vibrational wavefn of upper state (initial)
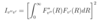
What are the singlet and triplet spin wavefns for a 2 e- atom? And give their symmetry
- state S = 0
- gives Ms = 0
- => singlet state
- Antisymmetric w.r.t exchange
- gives Ms = 0
- S = 1
- gives Ms = -1, 0, 1
- => triplet state
- Symmetric w.r.t exchange.
- gives Ms = -1, 0, 1
- spin-up × spin down cancels

What condition does symmetry of spin part of wavefn place on spatial part of wavefn? Why is ground state of helium a spin singlet?
- e-s are fermions hence total wavefn is antisymmetric
- if spin = antisymmetric then spatial = symmetric; vice versa
- e-s in ground state of helium have same n, l and m numbers
- so the spatial part is symmetric (since the antisymmetric part is 0)
- hence spin part has to be antisymmetric => spin singlet
What is meant by total collision cross-section and differential cross-section in particle scattering experiments?
- total (σT): effective area (normal to direction of incidence) provided by a target to an incoming projectile
- differential: particle flux cattered by each target nucleus into solid angle dΩ divided by incoming intensity
- flux = particles per unit time
- intensity = particles per unit time per unit area = flux per unit area
What is Beer-Lambert law?
- n = density
- l = length of cell
- I/I0 = 100% - %attenuation
- σT = total collisional cross-section
State the Pauli exclusion principle.
- Wave-functions are anti-symmetric w.r.t exchange of identical fermions
- and symmetric w.r.t exchange of identical bosons.
Why are (L + S) odd forbidden by the Pauli exclusion principle?
- If L is odd => odd pairing of e-s with the same l
- so there maybe an e- which doesn’t have a pair e- with the same n and l value
- therefore under exchange, the set up is now different
- => spatial wavefunction is antisymmetric
- forces the spin part to be symmetric.
- But L+S is odd so S is even
- spin wavefunction is antisymmetric
- if both spin and spatial are antisymmetric
- then total wavefunction is symmetric
- => forbidden by Pauli
When is the magnetic field in Zeeman effect considered strong?
Draw uncoupled LS diagram.
- If the splitting due to B field is bigger than the splitting due to LS coupling.
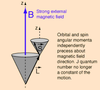
Sketch qualitatively the main features displayed in a typical X-ray spectrum

A diatomic molecule absorbs light in 3 different wavelength regions, what are the regions and the changes that give rise to the absorption of light.
- Microwave - Transitions between rotational levels only
- Infrared - Transitions between rotational and vibrational states together
- Ultraviolet - Transitions between rotation, vibrational and electronic states. So high energy as electronic energy level differences are much greater.
What are the steps needed to achieve laser action? Why is this not possible in a two-level system?
- 2 photons from stimulated emission have same frequency (coherent)
- each photon can stimulate further transitions => monochromatic waves coherent on a large scale
- stimulated emission to be dominant need population inversion
- but cannot be produced in 2 level system where population of state 2 is increased until N1 = N2 (saturated transition so absorption & stimulated emission are equally likely but stimulated emission cannot become dominant process)
- To obtain population inversion, need at least 3-level system
- atoms pumped with photon of frequency v13
- atoms accumulate in level 3 which spontaneously decays to level 2
- atoms accumulate in metastable level 2
- so levels 2 & 1 have inverted populations
- incoming v12 photons result in laser light at frequency v12
Give one example of a specific system which exhibits laser action showing how it meets requirements for laser action.
Ruby laser:
- Green light (550 nm) pumps Cr3+ ions in Ruby crystal (Cr3+ is impurity in Al2O3).
- Rapid (non-radiative) de-excitation to lower energy, metastable state => population inversion
- This can lase in red at 694 nm.
- Set-up requires > 50 % of ground state to be pumped ie large pumping power.
- Atoms in level 1 will absorb laser photons. This type of laser not very efficient.
How does PEP lead to exclusion for electrons with identical quantum numbers?
Consider the a and b that label the wavefuction to be a given set of quantum numbers n, l, m and n’, l’, m’.

State the Born-Oppenheimer approximation.
- Nuclei move much more slowly than electrons since they are much heavier => me/MN << 1
- Consider the nuclei as fixed and find the reduced mass of the system of nuclei.
- For simplicity, consider also ideal diatomic molecules.
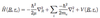
Selection rules for rotational transitions and energy difference
ΔJ = ±1
(for molecules with per. dip. mom. => can be rotated by external E field)
ΔJ = 0 => Possible for non-linear molecules
Nuclear motion
- Rotation - dictated by spherical harmonics
- Vibration - Radial eqn

Difference in intensity of rotational transitions for covalent and Ionic molecules
- Ionic molecules have a permanent electric dipole moment => obey ΔJ = ±1
- Purely covalent molecules, like homonuclears, have no electric dipole moment => ΔJ = 2 forbidden transitions which are much weaker.
- Ionic molecules have much stronger rotational transition intensities
Draw the energy level diagram and energy level spectrum for pure rotational transitions.
- Note that the spacing in the ENERGY SPECTRUM is 2B.
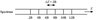
At which frequencies do rotational transitions occur?
Microwave
At which frequencies do vibration-rotational transitions occur?
Infrared.
What are the selection rules for vibration-rotation transitions?
- v is the vibrational quantum number
- For harmonic approximation:
Δv = ±1 , |J - J’| = 1
- Δv = ±1 comes from harmonic oscillator approx., and Δv = ±2,±3,±4,.. (overtones) are much weaker transitions.
Explain the branches that arise in rot-vib transitions.
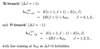
Give the total energy for an ideal diatomic molecule.
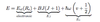
When is the total energy for an ideal diatomic molecule valid for real molecules?
What causes it to become invalid?
- v small: harmonic approximation is best near bottom of well
- J small: centrifugal distortion tends to stretch molecule and lower rotational energy.
- Rotational constant B depends on v. Since molecules vibrate, effective bondlength =/= Re => error when only considering rotation
- Potential V only approximately parabolic, for low v. For high v, corrections due to anharmonicity of potential needed.
Draw the rot-vib energy level diagram and spectrum.
Where is the line at Hbar*w0 missing?
- Since ΔJ = 0 is not allowed, the line at Hbar*w0 is missing.

Give the electronic transition selection rules
and which frequency range they occur in?
- Electronic transitions are always accompanied by vibrational and rotational.
- Selection rules: Δ Λ = 0, ±1 ΔS = 0, parity change g => u
- Since energy diff in electronic energy levels are much larger, transitions occur in ultraviolet.
What are molecular orbitals and electronic spectroscopic notation?
- Linear combination of H2+ orbitals
- Sum gives symmetric wavefunction, diff gives antisymmetric wavefunction.
- Electronic energy levels labelled by total electronic angular momentum Λ, similar to L in atoms. In the same way as with S, P, D, F,… we have for molecules Σ,
Π, Δ, Φ,…
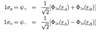
What are the electronic-vib-rot transition selection rules?
- Δv can take any value - vib energy levels
- ΔJ = 0, ±1 - rot energy levels
- Since bond length Re can change, B (rot. constant) can also change and we don’t get evenly spaced roational spectrum lines.
- Also, since En, Ev >> EJ - spreads electronic-vibration frequency into band of closely spaced lines.
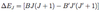
How are x-ray spectra produced?
- Bombard high Z atoms ( make up the anode) with energetic electrons from the heated cathode which are accelerated across the potential difference V between the anode and cathode => K.E = eV
Name 2 types of X-ray spectra
- Continuous x-ray spectra
- Characteristic x-ray spectra
Describe the formation of continuous x-ray spectra
- Fast moving electrons are deflected by the coulomb field of heavy atoms
- Charged particles emit radiation when accelerated (or decelerated)
- This is continuous background radiation => Bremsstrahlung (braking radiation)
- X-rays produced
- Limiting case when electrons brought to a stand still and all energy is given up => cut off wavelength
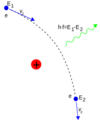
Describe the formation of characteristic x-ray spectra
- Some of the energetic electrons are able to collide with inner shell electrons with enough energy to cause ionisation of the electron.
- These electrons leave “gaps/or holes” behind which are filled by higher energy state electrons making the transition to the lower state.
- This results in the emission of characteristic x-rays

Describe what leads to laser action.
- When population of level 1 state is smaller than level 2, N1 < N2 - Photon more likely to be absorbed.
- If level 2 more populated than 1, N2 > N1 photon likely to stimulate emission.
- Atoms from emission goes on to cause further stimulated emission and cascade of photons produced - LASER
- Therefore, population inversion where N2 > N1 is a necessary requirement of laser action.
- However, population inversion is not possible in a 2 level system.
Describe laser action in a hypothetical 3 level system.
- Atoms pumped with photons of frequency ν13.
- Atoms accumulate in level 3 which decays spontaneously to level 2.
- Atoms accumulate in metastable level 2.
- Levels 2 and 1 have inverted populations.
- ν12 photons result in laser light at frequency ν12.

Discuss briefly 2 main types of bonding found in diatomic molecules explaining the interaxtions that lead to this bonding.
Give an example molecule for each.
- Covalent bonds - Sharing of electrons between atoms of similar electronegativity. H2 as example.
- Ionic bonds -Electron found predominantly close to one atom. Atoms have very diff. electronegativity. Normally occurs for alkali-halogen pairs, i.e. LiF, NaCl, etc.
Define electron affinity and ionisation potential.
- Electron affinity A - Energy required to add an electron
- Ionisation I - Energy to remove an electron (binding energy of valence electron)
- Calculating cost of removing electron from alkali atom and adding it to Halogen: e.g. NaCl
I(Na) - A(Cl)
Subtract since A is the energy gained by the Chlorine atom.
How can the bound energy levels of a hydrogen-like atom En = -Rinfinity Z2/n2 be modified to include effective nuclear charge, quantum defect and finite nuclear mass? How does quantum defect vary as a function of n and l, and why?
- change Z to Zeff
- Zeff = nuclear charge + charge of inner e-s
- since outer e- may have many e-s between it & the nucleus
- change n to neff = n - Δnl
- Δnl is quantum defect
- quantifies departure from hydrogenic behvaiour to account for shielding effects of inner e-s on nuclear attraction experienced by outer e-
- Δnl is quantum defect
- change Rinfinity to RM
- nucleus no longer assumed to be infinitely heavier than the e-
- Δnl increases with l and n
- since less nuclear attraction experienced for outer e- at high l
- so more hydrogenic like
- varies slower with n for a given l
- since less nuclear attraction experienced for outer e- at high l
Explain the origins of spin orbit interaction and Zeeman effect correction terms in the Hamiltonian for an atom in a B field (in terms of interaction between magnetic dipole moment and a magnetic field). What is the effect of each of the terms on energy levels of the atom?
- spin orbit correction term HLS
- due to internal atomic magnetic fields
- rest frame of e-, nucleus moves around it acting like an effective current loop => generates its own magnetic field
- e- has own magnetic dipole moment due to its spin
- magnetic field of nucleus interacts with magnetic dipole moment of e- so e- experiences a torque => energy cost depending on relative orientation of nucleus and e-
- hence energy correction term corresponding to HLS
- due to internal atomic magnetic fields
- Zeeman effect correction term HB
- magnetic moments due to spin & orbital angular momentum interact with external B field
- => potential energy VB (perturbation due to external B field) corresponding to HB
- magnetic moments due to spin & orbital angular momentum interact with external B field
- effect:
- LS causes splitting of terms to give levels
- B causes further splitting of these levels
What are the limiting cases where HLS >> HB and HLS << HB? Also which are the good quantum numbers (operators commute) in each case?
- HLS much greater => LS remain coupled & external B field is weak
- good quantum numbers: J, MJ, L, S
- HB much greater => strong external B field causes LS to uncouple
- good quantum numbers: L, ML?
Vector diagram for weak B field in Zeeman effect

Calculate separations of all the components of a state given the energy separation between highest and lowest energy levels in 0 magnetic field
- find J values
- ΔEmax given by Lande interval rule with sum of all the separations of components J1, J2, …, Jn (higher Js of separations where ΔJ = 1)
- ΔEmax = A(L,S) J1 + A(L,S) J2 + … + A(L,S) Jn
- => ΔEmax = A(L,S) Σi Ji
- obtain A(L,S)
- ΔEmax = A(L,S) J1 + A(L,S) J2 + … + A(L,S) Jn
- then separations of each individual component is
- ΔEi = A(L,S) Ji
Estimate magnitude of an external magnetic field that is considered strong
- when energy level splitting due to weak field = that due to LS
- ΔEweak field = gJMJμBB > smallest splitting (separations of components) due to LS coupling
- ignore MJ since only considering energy separation between levels
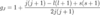
Which type of diatomic molecules undergo dipole-allowed vibrational transitions, and why? Give examples of these molecules
- heteronuclear molecules
- hence have some electronic distortion and so have electric dipole moment
- compared to linear molecules like H2 whose displacement is 0
- e.g. HCl and CO
Sketch Morse potential, marking De and Re, also include a parabolic potential that approximates the Morse potential near potential minimum
- Re = equilibrium bond length
- De = potential minimum (dissociation energy) = V(Re)
- What’s D0?

How to tell if potential is in harmonic form (Morse potential is approximately harmonic for small displacements from Re)?
- if V(R) is approximately parabolic => motion is approximately harmonic
- Taylor series expansion of V(R) about R=Re
- can neglect terms higher than quadratic
- at minimum dV/dR (at R=Re) = 0
- giving the eqn (to 2nd order)

How to calculate photon frequency between vibrational levels near bottom of potential well from spring constant of bond?
- ω2 = k/m usually
- for bonds, replace m with μ
- hence ω = sqrt (k/μ)
- remember mass of hydrogen = mass of proton
- and divide by 2π for answer in Hz unless stated to give in s-1
How does the spectrum of a
- one-electron atom
- rotating molecule
- vibrating molecule
depends on reduced mass of the system? Which one is most sensitive to isotopic changes?
- RM correction term for Rinfinity depends on μ
- E proportional to μ
- B depends on μ
- E proportional to 1/μ
- ω depends on μ
- E proportional to 1/sqrt(μ)
So rotating most sensitive to isotopic changes since E decreases rapidly for increasing μ
Explain each of the 5 terms in the Hamiltonian for H2+ molecular ion in atomic units.
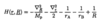
- 1st = sum of KE of each nuclei
- 2nd = KE of single e-
- 3rd = attraction between e- and nucleus A
- 4th = attraction between e- and nucleus B
- 5th = internuclear repulsion
- 2nd to 5th terms make up electronic Hamiltonian

What are the Schrodinger equations for electronic and nuclear motion in Born Oppenheimer approximation?
- terms with ∇Rψ(ri) do not feature in electronic Schrodinger eqn since electronic wavefn is assumed to be indt of changes in position of nuclei (nuclei positions fixed)

Spectroscopic notation for single electron

What further quantum numbers needed to completely specify state of electron in hydrogen?
What makes a magnetic field “strong” or “weak”?
What is the Hamiltonian for a molecule?

How to calculate average rotational constant B?
- Erot = BJ(J+1)
- calculate B for each transition
- and calculate the average B from those Bs
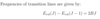
Converting between joules and cm-1.
- Divide through by hc to get cm-1
How do you expect the intensities of the rotational transitions in HD and HT to compare to those of rotational transitions in an ionically bound molecule, such as LiF?
How Stark effect in n=1 ground state of hydrogen differs from that in excited states with n>1?
- n=1 of H (only has s state) has atoms with no intrinsic dipole moment
- since H ground state is a non-degenerate state (has definite parity) & cannot possess an intrinsic electric dipole moment
- induced dipole moment by Eext
- VE = (-1/2)α(Eext)2 hence quadratic Stark effect
- ΔE = -E2ext(A+BM2J)
- n>1 have atoms with intrinsic dipole moment
- atoms have l-degeneracy i.e. all l levels for a given n have same energy
- ΔE = ∓3eEexta0
- new eigenstates of new H are also eigenstates of unperturbed H0 so no WD in mixing wavefns so energy is linear in Eext
Electric field difference moved through to be decelerated from vi to vf (stark effect)
- The stark energy change is equal to the change in kientic energy. Equate them to find the magnitude of the electric field, dF.
Draw an energy level diagram indicating dependence of energies of each of the four n=2 states in the presence of an applied E field along z. Label each state with its approximate wavefn expressed as Ψnlm
Give expression of the forces acting on Stark states of H atom in an inhomogenous E field
How to find most probable radius and mean radius of a (n,l) orbital given the wavefn?
- most probable radius from maximum of radial prob. distribution i.e. dPnl(r)/dr=0
- Include 4π in Pnl(r)=r2|Rnl|2 if wavefn is not normalised
- average radius
- integrate from 0 to infinity of rP(r)dr
What is the total number of states with same En i.e. total degeneracy wrt l and m?
- degeneracy wrt m is a feature of central potential (i.e. V = V(r)) which depends on |r|
- hence potential has spherical symmetry
- degeneracy wrt l is characteristic of 1/r potential

What is the Hamiltonian for N-electron atom?

When does central field model make most sense?
- most alkali atoms
- one loosely bound outer e- which is optically active
Why is spin singlet higher in energy than triplet state with same configuration?
- spin singlet e-s are closer together and experience stronger repulsion
- spin triplet keeps electrons apart (tend to avoid each other and Coulomb repulsion reduced)
- spatial separation is a result of spin orientation
What is the exchange force?
- behaviour of sign dependency on orientation of e- spins
- consequence of Pauli Principle which couples spin and space states (one depends on the other)
- Visualisation of spin-orbit coupling
- Orbital electron motion causes magnetic field within atom whose direction depends on L;
- Internal magnetic field causes torque on electron’s spin magnetic moment with direction determined by S;
- Torque doesn’t change magnitude of S, and reaction torque doesn’t change magnitude of L;
- Torques cause precessional motion of S and L with orientation dependent on each other;
- S and L precess about their sum J =L+S;
- Sz and Lz are not constants of motion.
What is the energy shift due to spin-orbit interaction? What is the Lande interval rule?

Total inelastic cross-section.
Total inelastic cross-section σij gives probability (effective area) for scattering changing internal state of target from level i to level j. Change accompanied by corresponding loss in (gain of) energy by the projectile. σij can be calculated from the Schr¨odinger equation.
For a perfect gas, number of atoms/molecules per unit volume is constant=Loschmidt’s number, L = 2.69x1025 (should be given)
- Q from ps: need to find n for Beer Lambert: n=(Lp)/p0*
- where p = pressure they give*
- p0= atomspheric pressure 105*
- Then sub what you get into Beer Lambert*
LOL what card, that is the card
Question: DCS depends on, in this case, cos2(theta):
means: Intensity=Ccos2(theta) where C=constant
to find constant they give you a way
e.g. we know at theta=45o and 2x109 intensity is collected.
you find C, then sum over cylindrical angles to get total beam not just at 45o
insert into Beer Lambert!!!!!!
no card


