Osmoregulation and Vasopressin System Flashcards
(34 cards)
Osmolarity
- the concentration of discrete solute particles in solution.
Isosmotic
having the same osmolarity as plasma (but can also refer to comparison with fluids other than plasma). The prefixes hypo- and hyper- refer to osmolarities below or above that of normal plasma.
Diuresis
urine flow above usual levels
Water Diuresis
increased urine flow due to decreased reabsorption of “free” water (i.e. water without solute).
Antidiuresis
- low rate of water excretion (usually <0.5 ml/min) as hyper-osmotic urine
Natriuresis
rate of urinary Na+ excretion above usual levels
Antinatriuresis
low rate of Na+ excretion
Regulation of ADH/Vasopressin Release
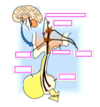
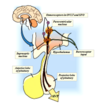
Regulation of Plasma [ADH] by Osmotic and Volume Stimuli

Collecting Duct Water Permeability is regulated by…
- ADH – Anti Diuretic Hormone also called vasopressin
- Activates the insertion of the water channel, aquaporin-2, into the apical membrane of the collecting duct
Formation of a concentrated urine: reabsorption of UREA in medulla
- Urea (and chloride) are passively moved along the proximal tubule, “dragged” through the membranes as water and salt move
- Most membranes with leaky tight junctions are permeable to urea
- As we progress from proximal to distal nephron, leaky “tight” junctions become tighter, now urea absorption is regulated
- Involved in concentrating mechanism, i.e. involved in the formation of a hyperosmotic urine (bright yellow!)
Proximal Tubule
- AQP-1 in both apical and basolateral membranes → very high transcellular hydraulic conductivity → a very small osmotic gradient (6 mOsm) can drive substantial water flow across these cells. Solute reabsorption establishes the osmotic gradient.
- Some water crosses the epithelium via the paracellular pathway (through “leaky” junctional complexes)
- Starling forces drive water from interstitium into peritubular capillary blood.
- Net effect: Tight coupling of solute and water transport across this highly water-permeable epithelium; 67% of filtered Na+ and water are reabsorbed in essentially isotonic proportions (iso-osmotic reabsorption)
Loop of Henle
•Descending Thin Limb – relatively high hydraulic conductivity (due to presence of AQP-1) allows water to move out of the lumen down an osmotic gradient.
-Note: There is only minimal active solute transport by these cells, so the osmotic gradient must be established by transport activity of other cells (Thick Ascending Limb)
•Thick Ascending Limb and Distal Convoluted Tubule: water-impermeable at all times (virtually no AQP present)
Collecting Duct
- Modulation of Water Permeability by Antidiuretic Hormone (ADH)
- Junctional complexes in the CD are “tight” –> no paracellular water reabsorption.
- In the absence of ADH: collecting cells are impermeable to water (AQP-3 and AQP-4 are present in the basolateral membrane, but no AQP is present in the apical membrane).
- In the presence of ADH: Clusters of AQP-2 are inserted into the apical membrane (cAMP-dependent) –> cells become water-permeable –> allows transcellular water reabsorption.
- Net effect: ADH can regulate water reabsorption by the collecting duct, which provides the opportunity to regulate water excretion (independent of solute excretion).
AQP-1
- Cloned from RBCs; Present in many tissues, especially the apical basolateral membranes of the proximal tubule and descending thin limb
- AQP-1 knockout mice have low proximal tubule water permeability
AQP - 2
- Apical membrane and intracellular vesicles in principal cells of the connecting tubule and collecting duct
- X-linked nephrogenic diabetes insipidus
AQP-3
- Basolateral membrane of principal cells in the connecting tubule and collecting duct
- AQP-3 knockout mice have low water permeability of the collecting duct
AQP-4
- Basolateral membrane of principal cells in the inner medullary collecting duct
- AQP-4 knockout mice have low water permeability of the collecting duct
Poorly-Reabsorbable Solutes Impede H2O Reabsorption
As a result of H2O reabsorption, filtered solutes that are poorly reabsorbable (“trapped” in the tubular lumen) become increasingly concentrated as they travel along the nephron. This process results in retaining osmotically obligated H2O in the tubular lumen, producing a diuresis.
- This type of diuresis is termed an osmotic diuresis, and is characterized by excretion of a largerthan-normal volume of urine that is rich in solutes.
- Poorly-reabsorbable solutes that produce an osmotic diuresis are termed osmotic diuretics.
- Examples:
1. Glucose (when the filtered load exceeds the Tm for glucose. Glucose not reabsorbed by the end of the proximal tubule is poorly reabsorbable in the remainder of the nephron, retaining water in the tubular fluid and resulting in an osmotic diuresis. Individuals with poorly-controlled diabetes mellitus typically exhibit a glucose-dependent osmotic diuresis.
2. Mannitol
Response to Water Intake
- The kidneys have a critical ability to vary relative proportions of solutes and water excreted in the urine, as needed to achieve solute and water balance.
- Excess TBW –> urine osmolarity can get as low as 50 mOsm/L
*Water deficit –> urine osmolarity can increase to 1200 mOsm/L
• These changes occur rapidly, in the absence of major alterations in plasma osmolarity, and without major changes in solute excretion.
Response to Water Intake – Formation of Hypo-osmotic (dilute) Urine
- Proximal tubule – “isosmotic” reabsorption of Na+ and water; fluid entering the loop of Henle is ≈300 mOsm/L
- Water leaves the water-permeable descending thin limb of the loop of Henle under the influence of increasing interstitial osmolarity (“medullary interstitial osmotic gradient”). Na+ is not reabsorbed here.
- At the bend of the loop, tubular fluid is concentrated due to water reabsorption (osmolarity is the same as the medullary interstitium).
- The entire remainder of the nephron is impermeable to water! Therefore, all water remaining in the tubule at the bend of the loop of Henle will be excreted in the final urine. However, solute reabsorption can occur.
- Some Na+ reabsorption occurs by diffusion as tubular fluid moves through the ascending thin limb into a progressively more dilute interstitium.
- The thick ascending limb reabsorbs Na+ (Na+-K+-2Cl– cotransporter), but no water. The tubular fluid is diluted (solute is removed, water remains) as it traverses this segment.
- Tubular fluid entering the distal convoluted tubule is hypo-osmotic (≈100 mOsm/L). • In the distal convoluted tubule, connecting tubule and collecting duct, additional active Na+ reabsorption (without water) further dilutes tubular fluid.
Net effect: Final urine can have an osmolarity as low as 50 mOsm/L (VERY DILUTE). Water can be excreted far in excess of solute! (This means that as little as 0.6% of filtered Na+ can be excreted together with up to 15% of filtered H2O!)
Response to Dehydration – Formation of Hyper-osmotic (Concentrated) Urine
- Requires ADH to render the distal tubule and collecting duct permeable to water.
- Processes from the glomerulus to the beginning of the distal tubule are identical to those that occur in formation of dilute urine.
- The hypo-osmotic tubular fluid entering the water-permeable (due to ADH) cortical collecting duct comes under the influence of a 300 mOsm/L (isosmotic) cortical interstitium. Water reabsorption occurs together with Na reabsorption. An isosmotic tubular fluid enters the medullary collecting duct.
- The medullary collecting duct is also water-permeable under these conditions (due to ADH), and it acts as an osmotic equilibrating device. Tubular fluid traveling through the medullary collecting duct comes under the influence of the medullary interstitial osmotic gradient. Water is reabsorbed down the very large osmotic gradient. Na+ (and urea) reabsorption also occur, contributing to the osmotic gradient.
- Maximum osmolarity of the final urine = the osmolarity of the inner medullary interstitium at the end of the collecting duct (near the papilla).
Net effect: Under the influence of ADH, a small volume of concentrated urine is formed. Compared to the formation of dilute urine, there is less water excreted (due to reabsorption in the distal tubule and collecting duct) but the same amount of solute is excreted (0.6% of filtered Na+).
Role of Urea in the Concentrating Mechanism
- In the presence of ADH, the inner medullary collecting duct becomes permeable to urea, which is passively reabsorbed into the interstitium by diffusion.
- Some of this urea diffuses into tubular fluid in the ascending thin limb, but it is effectively “recycled” as it is consequently reabsorbed again when it reaches the inner medullary collecting duct. •
Net effect: Urea recycling contributes about 40% of inner medullary solute, reinforcing the interstitial osmotic gradient under when concentrated urine must be formed (under the influence of ADH).


