Nephrotic Syndrome I and II Flashcards
Glomerulus
•Anastomosing capillaries lined by fenestrated endothelium, supported by mesangium, surrounded by Bowman’s capsule, with two layers of epithelium (visceral and parietal).

Glomerular Capillary Wall
- Thin layer of fenestrated endothelium.
- Glomerular basement membrane (lamina densa, lamina rara interna and lamina rara externa), composed mostly of Type IV collagen.
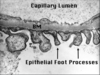
- Visceral epithelial cells (podocytes) – Interdigitating “foot” processes, separated by slit diaphragm (filtration slit).

Mesangium
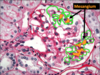
- Supports the capillary loops.
- Consists of cells and matrix

Bowman’s Capsule
•Lined by parietal epithelial cells.
Nephrotic Syndrome
Clinical condition related to dysfunction of glomerular podocyte. Four key components:
- Proteinuria > 3.5 gm/24 hours.
- Hypoalbuminemia
- Hyperlipidemia and lipiduria
- Edema
Acute Nephritic Syndrome
- Clinical condition associated with glomerular capillary dysfunction/inflammation (active glomerulonephritis).
- Main clinical features: Hematuria, proteinuria, increased blood pressure, edema, increased serum creatinine, and active urinary sediment.
- Active urinary sediment – Red blood cells and casts (made of red blood cells, white blood cells and/or epithelial cells) detected in the urine; indicates active glomerulonephritis
•Rapidly progressive glomerulonephritis - Severe form of acute nephritic syndrome with rapid rise in serum creatinine (renal emergency).
Persistent Asymptomatic Urine Abnormalities
•Usually subnephrotic range proteinuria (< 3.5 gm/24 hours) or persistent/recurrent microscopic hematuria.
Renal Failure
•Elevated serum creatinine.
- Acute – Glomerular, vascular or tubulointerstitial disease.
- Chronic – Advanced renal disease, usually irreversible, due to a variety of primary diseases.
Clinical Presentations Leading to Renal Biopsy
- Nephrotic Syndrome
- Acute Nephritic Syndrome
- Persistent asymptomatic urine abnormalities
- Renal Failure
Three Modalities of Renal Biopsy Examination
- Light Microscopy
- Immunofluorescence Microscopy
- Electron Microscopy
•All samples must have cortex with glomeruli
Light Microscopy
- Multiple level sections of formalin-fixed tissue cut and mounted on glass slides.
- Basic stain: Hematoxylin and Eosin (H&E)
•General stain, good for inflammatory cells
- Special stains:
•Periodic Acid Schiff (PAS)
- Mesangium, basement membranes
•trichrome
- Fibrosis, necrosis
•Jones silver stain
- Basement Membranes

Immunofluorescence Microscopy
- Used to detect presence of immunoglobulin and complement proteins.
- Multiple level sections of frozen tissue cut and mounted on glass slides.
- Proteins detected: IgG, IgM, IgA, C3, C4, C1q, fibrinogen, albumin, kappa light chain, and lambda light chain.
• Immunofluorescence Microscopy (IF) Antibodies used:
– Immunoglobulins:
- Heavy chains: IgA, IgG, IgM
- Light chains: kappa, lambda
– Complement: C3, C4, C1q
– Fibrin/fibrinogen
• Marker of severe injury (necrosis, crescents)
– Albumin
• Good barometer for background staining - “Stickiness” factor
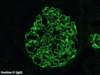
Electron Microscopy
- Transmission electron microscope is used to examine ultrastructure of renal tissue.
- Tissue fixed and embedded in hard epoxy resin; ultrathin sections cut using diamond blade; sections mounted on a grid and examined using electron microscope.


Three Proposed Mechanisms of Immune Complex Formation
•Some forms of renal disease are caused by antigen-antibody complexes.
- Antigen-antibody complexes form in the blood, circulate to the kidney and are then deposited into renal tissue.
- A circulating antigen is first deposited into the kidney, and the recognizing antibody then binds to the planted antigen.
- A protein normally present in renal tissue acts as an auto-antigen, and the recognizing antibody binds to this intrinsic renal protein.
Mechanisms of Renal Injury
- Antigen-antibody complexes activate the complement cascade.
- Some results of complement activation:
a. Elaboration of cytokines and chemokines.
b. Recruitment of inflammatory cells.
c. Damage to renal tissues from cell lysis, actions of inflammatory mediators, activation of digestive enzymes, etc. - Inflammatory type of injury more severe in diseases that cause acute nephritis vs nephrotic syndrome.
Pathophysiology of Nephrotic Syndrome
- Glomerular Proteinuria
- Hypoalbuminemia
- Edema
- Hyperlipidemia and Lipiduria
Glomerular Proteinuria
– Increased filtration of macromolecules across glomerular capillary
– Due to abnormalities in podocyte
– Albumin – Principal urinary protein
- Clotting inhibitors
- Transferrin
- Vitamin D binding protein

Hypoalbuminemia
– Consequent to urinary albumin loss
– Hepatic albumin synthesis increases but is unable to sufficiently replete serum levels
– Serum albumin levels are low
• Usually <2 g/dl (nl 3.5-5.5 g/dL)
Edema
– Hypoalbuminemia causes egress of fluid into interstitial space
• Due to decreased plasma oncotic pressure
– Stimulation of the Renin-Angiotensin system
- aldosterone release causing marked sodium retention
- sympathetic stimulation
- reduced natiuretic peptide release
– Soft dependent, pitting edema
Hyperlipidemia and Lipiduria
• Hyperlipidemia
– Decreased oncotic pressure stimulates hepatic lipoprotein synthesis
- Manifests as hypercholesterolemia, hypertrigliceridemia
- Lipiduria
– Lipid in urine becomes entrapped within protein material in renal tubules “lipid casts”
– Enclosed by cytoplasmic membrane of degenerated cells = “oval fat body”
• Cholesterol in oval fat bodies appear as maltese crosses under polarized light

Complications of Nephrotic Syndrome
A. Altered coagulation
- Thromboembolism - Typically seen with >10 grams of proteinuria.
a. Increased hepatic production of procoagulation factors.
b. Anticoagulant factors lost through glomerular capillaries (anti-thrombin III).
c. Concomitant volume depletion from diuretic therapy + reduced oncotic pressure in the vasculature leads to hemoconcentration and increased platelet aggregation.
d. Thrombus formation
i. Predilection for renal vein thrombosis (likely due to loss of fluid across the glomerulus and ensuing hemoconcentration in the postglomerular circulation).
B. Infection
- Due to immunoglobulin loss in the urine.
- Common infections: Staphylococcus and Streptococcus pneumoniae
Generalized Treatment of Nephrotic Syndrome (Non specific Therapy)
A. Renin-angiotensin system: All should be treated with ACE inhibitors (ACEi) and or angiotensin receptor blockers (ARB) to reduce intraglomerular capillary pressure and reduce proteinuria.
B. Diuresis: Loop diuretics for edema, low sodium diet (<2g/day).
C. Strict blood pressure control: Goal <130/80 mm/Hg.
D. Statin: Treatment of hyperlipidemia (Statin = HMG-CoA reductase inhibitor).
Causes of Nephrotic Syndrome
- Primary Glomerular Diseases
- Membranous Nephropathy
- Minimal Change Disease
- Primary Focal Segmental Glomerulosclerosis
- Idiopathic/Autoimmune Membranoproliferative Glomerulonephritis - Secondary Systemic Diseases
- Diabetic Nephropathy
- Amyloidosis
- Systemic Lupus Erythematosus
Minimal Change Disease (MCD) - Epidemiology
•Primary glomerular disease
- Most common cause of nephrotic syndrome in children; (90% in children 10 years with increased percentage typically FSGS).
- Accounts for 10-15% of nephrotic syndrome in adults.
Minimal Change Disease (MCD) - Etiology
•Primary glomerular disease
- Idiopathic
- Drugs – NSAIDs
- Neoplasm – Hodgkin’s disease, lymphoma, leukemia
Minimal Change Disease (MCD) - Pathogenesis
•Primary glomerular disease
- Exact cause unknown; key feature: Podocyte injury
a. Systemic T cell dysfunction thought to result in production of a glomerular permeability factor that injures podocytes leading to foot process effacement and proteinuria. - May follow respiratory infection or viral illness.
Minimal Change Disease (MCD) - Renal Biopsy Findings
•Primary glomerular disease
- Light Microscopy
a. Normal appearance (i.e. “minimal” changes) - Immunofluorescence – Negative
- Electron Microscopy
a. Diffuse effacement of podocyte foot processes
b. No immune complexes; normal glomerular basement membrane

Minimal Change Disease (MCD) - Clinical Course
•Primary glomerular disease
- Historically, untreated MCD was associated with risk of mortality due to infection and risk of thromboembolism.
- Generally responsive to steroid therapy (90% of children respond) with good prognosis; no progression to chronic renal disease.
- Patients may have acute renal failure due to tubular injury (acute tubular necrosis)
Minimal Change Disease (MCD) - Treatment
•Primary glomerular disease
- All should receive nonspecific therapy outlined above (section VII).
- High dose steroids (prednisone).
- Cyclophosphamide in patients who are steroid-dependent (i.e. steroids cannot be stopped without relapse in nephrotic syndrome).
- Cyclosporine in patients who are resistant (no or poor response) to steroids; consider re-biopsy as diagnosis may be FSGS.
Focal Segmental Glomerulosclerosis (FSGS) - Epidemiology
•Primary glomerular disease
- Most common cause of nephrotic syndrome in adults (~ 35% of all biopsies for nephrotic syndrome).
- Second most common to minimal change disease in children.
- Increased incidence in African-Americans and males.
Focal Segmental Glomerulosclerosis (FSGS) - Etiology
•Primary glomerular disease
- Primary - Idiopathic and genetic/familial
a. Familial forms are associated with genes encoding slit diaphragm proteins in the foot process of the podocyte, including nephrin, podocin, TRPC6, alphaactinin-4. - Secondary – Heterogeneous, occurs in many forms of renal injury and systemic disease.
a. Special associations: Loss of renal mass, obesity, HIV, Sickle Cell disease, drugs.
Focal Segmental Glomerulosclerosis (FSGS) - Pathogenesis
•Primary glomerular disease
- Primary – Mechanisms of podocyte injury:
a. Immune dysregulation - Systemic T-cell dysfunction, similar to MCD (same disease continuum but reduced responsiveness to therapy).
b. Genetic mutations of podocyte proteins (as above)
c. May involve presence of a circulating toxin which is supported by the rapidity of recurrent disease following renal transplant. - Secondary – Mechanisms of podocyte injury:
a. Hyperfiltration and increased glomerular capillary hypertension (in reduced renal mass, obesity, Sickle Cell disease).
b. Direct injury to the podocyte from virus (HIV).
Focal Segmental Glomerulosclerosis (FSGS) - Renal Biopsy Findings
- Light Microscopy
a. Focal glomeruli show sclerosis (scarring) of a segment of the glomerular capillary tuft.
b. Sclerotic lesions may have accumulation of collagen/matrix material, foam cells and/or proteinaceous material (hyaline). - Immunofluorescence
a. No specific immune complex deposition.
b. Non-specific trapping of immunoglobulins and complement (usually IgM and C3) in areas of sclerosis. - Electron Microscopy
a. No immune deposits.
b. Obliteration of a segment of the glomerulus by accumulation of matrix, cells and plasma protein material (hyaline).
`c. Diffuse podocyte foot process effacement in primary FSGS; variable/patchy effacement in secondary FSGS.

Focal Segmental Glomerulosclerosis (FSGS) - Clinical Course
•Primary glomerular disease
- Primary FSGS may present with acute or insidious onset.
a. Hematuria in 30% of patients, hypertension, variable degrees of reduced function. - Secondary FSGS always presents with insidious onset.
a. Presents more often with nephrotic range proteinuria (>3.5 g) rather than full nephrotic syndrome. - Untreated primary FSGS follows a progressive course to ESRD, rate of spontaneous remission 10g), reduced renal function (elevated creatinine), and presence of tubulointerstitial fibrosis.
- Risk factors for progression include high level proteinuria (i.e. >10g), reduced renal function (elevated creatinine), and presence of tubulointerstitial fibrosis.
- Primary FSGS may recur after transplant, sometimes within days.
a. See diffuse podocyte injury by EM first; then sclerotic lesions appear by light microscopy as recurrent disease progresses.
Focal Segmental Glomerulosclerosis (FSGS) - Treatment
•Primary glomerular disease
- Primary FSGS – Treatment similar to MCD
a. All should receive nonspecific therapy outlined above (section VII).
b. High dose prednisone.
- Less likely to respond vs MCD.
c. Cyclophosphamide or cyclosporine in patients who are steroid dependent and/or resistant.
d. If no response to above therapies, may have familial FSGS with mutations in slit diaphragm proteins. - Secondary FSGS
a. Nonspecific therapy -
ACE inhibitors and angiotensin receptor blockers.
b. Treatment of underlying disease (i.e. HIV)
Membranous Neuropathy - Epidemiology
•Primary glomerular disease
- Second most common cause of nephrotic syndrome in adults.
- Occurs in all ethnic groups, most common in men >40 yrs.
Membranous Neuropathy - Etiology
•Primary glomerular disease
- Primary/idiopathic (70%) and secondary (30%) forms.
- Associations in secondary membranous:
a. Drugs (penicillamine, captopril, gold, NSAIDs)
b. Malignancy (carcinomas, melanoma)
c. Systemic lupus erythematosus
d. Infections (Hepatitis B, lesser extent Hepatitis C)
Membranous Neuropathy - Pathogenesis
•Primary glomerular disease
- Immune complex mediated disease
a. Primary – Often due to circulating IgG antibodies directed against antigen expressed on the podocyte foot process (antigen = Type-M phospholipase A2 receptor).
b. Secondary - Antigens = Viral proteins, tumor proteins, drug related substances, etc. - Immune complex formation activates complement cascade.
a. Assembly of C5b-9 (membrane attack complex) inserts into podocyte plasma membrane resulting in complement-mediated podocyte injury. - Proteinuria – Due to podocyte foot process effacement (dysfunction of slit diaphragm) and loss of podocytes (apoptosis, necrosis, and detachment from the underlying basement membrane).
Membranous Neuropathy - Renal Biopsy Findings

- Light Microscopy
a. Diffuse thickening of the glomerular capillary loops.
b. Small “spikes” of glomerular basement membrane material sometimes visible by Jones silver stain. - Immunofluorescence
a. Fine granular immune deposits along the glomerular capillary walls.
b. Usually IgG, C3, kappa and lambda. - Electron Microscopy
a. Numerous, small electron dense immune deposits along the subepithelial aspect of glomerular basement membrane.
b. Reactive “spikes” of glomerular basement membrane material form between deposits.
c. Diffuse podocyte foot process effacement.

Membranous Neuropathy - Clinical Course
•Primary glomerular disease
- Spontaneous remission occurs in up to 30% patients at 5 years; spontaneous partial remission (<2 grams proteinuria/day) occurs in up to 40% patients at 5 years
- Progression to ESRD in 40% at 15 years if untreated.
- Risk factors for progression:
a. Older age of onset, male > females, increased serum creatinine, >8 grams of proteinuria at presentation, presence of tubulointerstitial fibrosis on biopsy
Membranous Neuropathy - Treatment
•Primary glomerular disease
- All should receive nonspecific therapy outlined above
- Immune-targeted therapy if there are risk factors for progression or patient remains symptomatic on ACEi/ARB/loops/diet.
a. Cyclophosphamide + prednisone administered over 6 months.
b. Cyclosporine + low dose prednisone as an alternative to those who fail or cannot tolerate cyclophosphamide.
c. Rituximab (monoclonal antibody to CD20) will likely replace Cyclophosphamide as first-line treatment
Diabetic Neuropathy - Epidemiology and Clinical Presentation
•Secondary - systemic disease
- Most common systemic illness to cause nephrotic syndrome and most common cause of ESRD in the US.
- Occurs in both Type I and Type II diabetes, often with genetic susceptibility.
- Common in Hispanic, Native American and Black populations.
Diabetic Neuropathy - Pathogenesis
•Secondary - systemic disease
- Metabolic – Hyperglycemia and pro-inflammatory mediators cause biochemical derangements of glomeruli leading to increased synthesis of matrix proteins and pro-fibrotic growth factors.
a. Nonenzymatic glycosylation (covalent binding of glucose) to proteins in glomerulus contributes to glomerulopathy. - Hemodynamic – Hyperfiltration leading to increased glomerular capillary pressure and glomerular hypertrophy.
Diabetic Neuropathy - Renal Biopsy Findings
•Secondary - systemic disease
- Light Microscopy
a. Glomeruli – Diffusely thickened glomerular basement membranes, increased mesangial matrix leads to formation of large nodules comprised of matrix material (Kimmelstiel-Wilson nodules).
b. Tubules/interstitium – Thick tubular basement membranes, progressive fibrosis.
c. Vessels – Intimal sclerosis of arteries (arteriosclerosis); hyalinosis of arterioles. - Immunofluorescence
a. No specific immunoglobulin or complement deposition.
b. Pseudolinear staining of glomerular and tubular basement membranes with IgG and albumin.
i. Due to “stickiness” of basement membranes. - Electron Microscopy
a. Diffuse thickening of glomerular basement membranes due to increased amount of lamina densa.
b. No immune deposits.
c. Increased mesangial matrix and mesangial cells, often forming nodules of matrix material.
d. Variable podocyte foot process effacement (more severe in advanced disease).

Diabetic Neuropathy - Clinical Course
•Secondary - systemic disease
- Patients present with proteinuria:
a. Early - Leakage of small amounts of albumin (microalbuminuria), often asymptomatic.
b. Late – Overt proteinuria with development of nephrotic syndrome. May take 10-20 years to develop. - Progression depends on blood sugar control. In many patients, progressive scarring leads to ESRD.
Diabetic Neuropathy - Treatment
•Secondary - systemic disease
- Nonspecific treatment outlined above (section VII).
- Strict blood sugar control – Oral hypoglycemics and/or insulin.
- Kidney-pancreas transplant – Definitive treatment for type I DM.
Amyloidosis - Definition and Epidemiology
- Group of diseases characterized by extracellular deposition of beta-sheet fibrils (abnormally folded protein) within kidney
- Cause of nephrotic syndrome in adults.
Amyloidosis - Pathophysiology
•Secondary - systemic disease
- Many different types, most important for renal disease are Primary (AL) and Secondary (AA).
a. AL (primary) – Seen in patients with plasma cell myeloma; the abnormal protein consists of monoclonal immunoglobulin light chains (often lambda).
b. AA (systemic reactive) – Seen in patients with chronic inflammatory diseases (i.e. rheumatoid arthritis, inflammatory bowel disease, hepatitis, chronic pyogenic infections including IV drug users).
i. Protein - Serum amyloid A protein
Amyloidosis - Renal Biopsy Findings

•Secondary - systemic disease
- Light Microscopy
a. Amorphous, pale eosinophilic material irregularly distributed in mesangium and along glomerular capillary loops.
b. Also can be present in vessels and interstitium.
c. Special stain: Congo red – Amyloid stains salmon or red color and shows apple green birefringence under polarized light. - Immunofluorescence
a. Variable; may see light chain restriction if AL type (i.e. staining only for kappa without lambda, if the amyloid consists of kappa light chains). - Electron Microscopy
a. Deposition of haphazardly arranged fibrils within the mesangium and glomerular basement membrane.
b. Variable podocyte foot process effacement.

Amyloidosis - Clinical Course and Treatment
•Secondary - systemic disease
- Poor prognosis, progression to end stage renal disease is common.
- Treatment – Nonspecific therapy outlined in Section VII should be initiated in primary and secondary amyloidosis.
a. Primary – Chemotherapy, peripheral blood stem cell transplant.
b. Secondary – Treat the underlying disease.

Amyloid

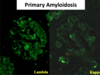
Primary Amyloidosis


