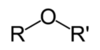Organic 1 Flashcards
Hydrazine
N2H4

Why are alcohols soluble in water?
They can H bond with water
Cycloalkanes and ring strain
- How much ring strain do big, small, bicyclic & monocyclic cycloalkanes have?
- Cycloalkanes w/ LESS than 6 C’s have increasingly more ring strain
- Really BIG (10+) rings have enough freedom to approximate 109.5º
- BICYCLICring systems haveMOREring strain thanMONOcyclic rings
1º,2º,3º OH groups have 1,2, and 3 ___ ___ which do what to charge?
What does this mean?
Have 1,2, and 3 donating groups to destabilize charge
this is why 3º is less acidic than 1º
Hückel’s Rule (for aromaticity)
to exhibit aromaticity
a ring must have EXACTLY __+__ electrons
to exhibit aromaticity
a ring must have EXACTLY: 4n+2π electrons
From what I’ve gathered, “n” is just 1 (at least it is for benzene…( 4(1) + 2π= 6 )

Hemiketal

Alkenes are _____s!
Why?
Nucleophiles!
- π bonds are e’ dense and will attach E:philes
- forming a new bond to one of the C’s
- and leaving a CCI on the other
- CCI then attacked by a Nu:
What does an SN2 rxn look like?

Rank BOND REACTIVITY for:
- Single bonds
- Double bonds
- Triple bonds
triple>double>single
more reactive bc of π bonds
3º ROHs can only be oxidized into ___?
PSYCHE!
They cant be oxidized at all!
1º ROH can be oxidized into?
1º ROH⇒Aldehydes⇒Carboxylic Acids
Alcohols are less/more acidic than water
- Rank alcohol acidity
LESS acidic than water
- ROH acidity increases from:
- (most acidic) 1>2>3 (least acidic)
Imine

Hybridization:
sp3, 1 LP
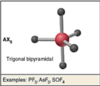
Trigonal Planar

Hybridization:
sp3d, 2 LPs
T-shaped

What is a racemic mixture a mixture OF?
50/50 mix of R and S enantiomers
Anomers
- If anomeric OH/OR group and CH2OH group are on opposite sides of the ring, what kind of anomer is it?
ALPHA anomer
on the “Alpha-site side”
Alcohols behave as either: ___s or as a ___
1) Nucleophiles
* (the lone pair on oxygen acts as a Lewis base)
2) Lewis acids
- ..when they are oxidized to carbonyl groups
- the oxygen ACCEPTS A PAIR OF ELECTRONS (Lewis Acid) from the O-H bond as the proton is abstracted
Hybridization:
sp3d2, 1 LP
Square Pyramidal

Are Alkyl substituents strong or weak e’ donating groups?
weak
Absolute conformation (__ & __)how do you assign priority to 4 R groups?
Assign priority starting with:
HIGHEST MW
(heavier=higher priority, i.e. 4)
(lighter, like H=lower priority, i.e.1)
If 1–>3 is clockwise=R
If 1–>3 is counter clockwise =S
Gringard Synthesis:
Describe the 2 steps (using R-MgBr)
1) Mg has VERY LOW ELECTRONEGATIVITY
- so the R group in R-MgBr attacks the electrophilic carbonyl C
- Occurs in single step
- kicking e’s in C=O bond onto the oxygen
2) (-) charged oxygen is protonated
* yielding an alcohol
What are diastereomers?
Have same MF & connectivity (like steroisomers)

- are NON-identical, NON-superimposable*
- mirror images*
Amine