EQUATIONS Flashcards
(91 cards)
Circuits
RESISTANCE
(of a material used as a resistor in a circuit)
UNITS=?
R=ρL/A
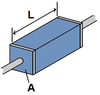
UNITS: Ω (Ohms)
- ρ=resistivity of the material
- L=length of material
- A=cross-sectional area
INTENSITY of a wave
=waves/m2
VELOCITY of a wave
V=λf
VOLTAGE in a circuit (3)
V=PE/q
V=Ed
V=Kq/r
KINETIC ENERGY
KE=½mv2
HARMONICS
FOR:
- String or pipe with MATCHING ends–
- both nodes or antinodes
- String or pipe open at ONE end –
- with one node and one antinode
λ=?

λ = 2L/n
λ = 4L/n
Snell’s Law
n1sinθ1 = n2sinθ2
Young’s Double Slit Experiment
x = λL/d
- x is the distance between fringes
- λ is the wavelength of light used
- d is the distance between the two slits
- L is the distance between the “double slit” and the final screen
TORQUE (3)
T=Fl
T=mgl
T=Frsinθ
GRAVITATIONAL PE
IN SPACE
PEgrav= - Gmm/r
PEELASTIC
PEelastic=½kx2
PEELECTRICAL (3)
PEelec=Kqq/r
PEelec=qEd
PEelec=qV
POTENTIAL ENERGY STORED IN A CAPACITOR (3)
PEcapacitor=½QV
PEcapacitor=½CV2
PEcapacitor=½Q2/C
MECHANICAL ENERGY (ME)
ME=KE+PE
WORK (2)
W=Δ ENERGY
W=Fdcosθ
RAMPS
Fm=mg (h/d)
- h is the height of the ramp
- d is the distance along its hypotenuse
LEVERS
Fm=mg( L1 / L2 )
- L1 is the lever arm for the mass
- L2 is the lever arm for the applied force
PULLEYS
Fm= mg / ( # of vertical ropes directly lifting the mass)
POWER (4)
P=ΔE/t
P=W/t
P=Fdcosθ
P=Fvcosθ
HYDRAULIC LIFTS (2)
Fm=mg (h1/h2)
Fm=mg (A1/A2)
h1=distance traveled by the large plunger
h2=distance traveled by the small plunger
A1 =cross-sectional area of the small plunger
A2 cross-sectional area of the large plunger
FORCE FOR A CONSTANT ELEC. FIELD
F=qE
FORCE FOR A POINT CHARGE ELEC. FIELD
F=Kqq/r2
<span>(Coulomb’s Law)</span>
STRENGTH OF FIELD (“E”) FOR A CONSTANT ELEC. FIELD (2)
E=F/q
E=V/d
STRENGTH OF FIELD (“E”) FOR A POINT CHARGE ELEC. FIELD
E=Kq/r2