Optional module - Diabetes and Obesity Flashcards
When are in-uteros at risk of obesity?
Babies that are born smaller than usual
Have + risk of obesity and CVD later in life
When are children at risk of adult obesity?
If overweight under 5 years old
+ risk if one or both parents are overweight
When are adolescence at risk of adult obesity?
If overweight when adolescence
When are adult females at risk of obesity?
- Pregnancy
- Oral combined pill
- Menopause
How does male weight change overtime?
- Progressive increase over the decades until 6th decade
- Progressive reduction in energy expenditure as age
- Stabilises between 55-64 then slowly declines
What other factors affect weight?
- Sleep deprivation
- Smoking cessation (4-5kg on average)
- Drugs:
- Antidepressants
- Antiepileptic drugs
- Diabetes drugs
- Beta blockers
- Glucocorticoids
What illnesses can affect weight?
- Hypothyroidism (low energy expenditure)
- Polycystic ovarian syndrome (50% obesity rates)
- Cushing’s syndrome (excess endogenous glucocorticoid)
- Hypothalamic obesity (severe uncontrolled increase in appetite)
What are general potentiual causes of obesity?
- Excess calorie intake
- Genetics, intrauterine environment and post-brith environment
- Ethnic differences
- Socioeconomic differences
What are problems with melanocortin-4 receptor deficiency?
Associated with early onset obesity and taller than average height
What are problems with POMC gene defects?
- Infant adrenal crisis
- Early onset obesity from hyperphagia caused by ‘alpha’ MSH deficiency
What is the function of leptin?
What are problems with leptin deficiency?
Informs brain of extent of fat stores
Deficiency = hyperphagia and obesity
What is Berardinelli Seip syndrome?
Leptin deficiency
Leads to no fat storage in body
What is Prader Willi syndrome?
- Genetic abnormalities on long arm of chromosome 15
- Neonatal hypotonia and cryptorchidism
- Hypothalamic dysfunction:
- Hunger with lack of satiety
- Obesity
- Low sex hormones and growth hormone
- Cognitive and behavioural challenges
What is Bardet Beidl syndrome?
- Autosomal recessivie disorder
- Obesity
- Intellectual disability
- Small male testes
- Retinal dystrophy
- Polydactyly
Where is the worst place to store fat and why?
What is the waist-to-hip ratio for this?
In the abdominal region
- Associated with + risk of CVD
- Apple shaped figure
Women = >0.85
Men = >1
What is increased visceral fat associated with?
What does comparision of normal and T2D CT look like?
Metabolic syndrome:
- hypertension risk
- hyperlipiodaemia risk
- impaired glucose tolerence progressing to type 2 diabetes risk
- CVD

Why is visceral fat a problem?

What are the main associated health risks with obesity?
- •Diabetes
- •Hyperlipidaemia
- •Hypertension
- •CVD
- •Heart failure
- •Atrial fibrillation
- •Stroke disease
- •Venous thrombosis
What are the musculoskeletal risks with obesity?
- Osteoarthritis
- Gout
What are the gastrointestinal risks with obesity?
- Hepatic disease
- NASH
- Cirrhosis
- Gall stones (Cholelithiasis)
What are the renal risks with obesity?
- Chronic kidney disease
- Renal stones
- Urinary incontinence
What are respiratory risks with obesity?
Sleep apnoea
What are the 2 main drugs used for weught loss in EU?
Orlistat - 60-120mg / day - Oral - intestinal lipase inhibitor, reducing fat absorption by 30%
Liraglutide - 3mg / day - SC injection - GLP-1 agonist, reducing hunger and delaying gastric emptying
What are the 3 types of surgery fpr weight loss?
Gastric bypass
Gastric banding
Duodenal bypass

What should glucose levels be and what are the dangers when out of this range?
- Normal circulating [glucose] is 4-5 mM.
- [glucose] below ~4 mM causes problems:
- [glucose] < 1-2 mM and brain cannot sustain activity
- brain is obligate glucose utilising tissue
•[glucose] above 7-8 mM is also dangerous:
- glycation of proteins
- peripheral and CNS neuron damage
What are the 3 main sources of glucose?
- Glycogenolysis
- Gluconeogenesis
- Diet
What are the properties of glucose uptake?
- Mediated by series of glucose transporters
- Glucose transporters = proteins in cell surface
- Channels form which allow transport across membrane
What is the main GLUT and why is it different from the others?
GLUT 4
Is the only insulin-dependent one
1-5 = don’t require ATP
What is the purpose of kinases in cells during glucose sensing?
Remove GLUT2 transporter from cell once glucose is in the cell

What is the order of events of glucose sensing in the brain of neonates and adults?

What 2 things directly cause insulin release in pancreatic ‘beta’-cells?
+ calcium ion concentration
G coupled protein binding

What are the properties of the insulin receptor?
- Is a tetramer (has 4 subunits)
- Beta subunits are in the membrane
- Alpha subunits are above membrane
- Subunits are attached by disulphide bridges
- Kinase domains phosphorylase other proteins

What are the early events of insulin signalling?
- Insulin binds as a monomer
- 2 identical binding sites per receptor
- Receptors are activated by conformational change from insulin binding
- ‘Beta’-subunit tyrosine kinase domains trans-phosphorylate one-another

What are the second early events of insulin signalling?
- Phosphorylated insulin receptor cytoplasmic domain acts as docking site for various IR effectors
- IR effects contain specific phosphotyrosine (pY) binding domains
- PTB and SH2 domains bind pY motifs
- IR tyrosine phosphorylates IRS, Shc and SH2 proteins

What are the third early events of insulin signalling?
- Phosphorylated IRS proteins recruit critical signallinh protein complex PI3K
- PI3K = heterodimeric enzyme composed of p85 and p110 subunits
- p110 = catalytic
- p85 = alpha and beta isoforms
- p110 = alpha, beta, gamma and δ isoforms
- PI3K phosphorylates PIP2, creating PIP3
- PIP3 acts as docking site for proteins containing PH domains
- PTEN antagonises PI3K and converts PIP3 back to PIP2
What are the fourth early stages of insulin signalling?
- Phosphorylated IRS proteins recruit Grb2
- SH2 and 3 domains
- Grb2 recruits and activates SOS
- GEF interacts with Grb2 SH3 domains
- SOS stimulates activity of Ras GTPase

What are the later events of insulin signalling?
- Kinases relay IR signalling downstream to effectors
- PKB = + important kinase
- pH domain
- Pro-survival/growth
What switch off insulin signalling?
Phosphotases
Kinases
What is GLUT4 and its importance?
A high affinity glucose transporter
- Predominantly expressed in muscle and adipose (muscle = 90% insulin dependent glucose uptake)
- Mediates insulin-dependent glucose uptake
- Predominantly intracellular in insulin absence (stored in GLUT4 storage vesicles (GSVs))
- Re-distributed to plasma membrane in insulin-dependent fashion
What are the results of insulin signalling in the liver?
- Gluconeogenesis inhibited by PP1 activation and phosphofructokinase-2 activation
- glycolysis
- glycogen synthesis
- cholesterol synthesis
- FA and TAG synthesis
- VLDL synthesis
- FA oxidation
- protein synthesis rate
What are the results of insulin signalling on muscle?
- GLUT4 in PM
- glycolysis
- glycogen synthesis
- FA oxidation
- TAG uptake
- protein synthesis rate
What are the results of insulin signalling on adipose?
- GLUT4 in PM
- lipolysis
- FA and TAG synthesis
- plasma TAG clearance
- protein synthesis rate
What does your BMI have to be to class you as obese?
> 30
How does the body deal with excess energy?
- Converting it to fat and storing it in adipose tissue
- Burnt by extra exercise
- Diverting it into heat production (in brown adipose tissue - UCP1)
What events occur during body mass homeostasis?
- Adipocyte releases peptides that signal the brain centre that inhibits eating behaviour and increases motor activity
- Leptin stimulates region that suppresses appetite
- fatty acid synthesis
- fatty acid degradation
- Leptin stimulates sympathetic nervous system
- Leptin inhibits fatty acid synthesis and activates fatty acid oxidation in liver and muscle
What is adiponectin and it’s function?
Peptide hormone, produced by adipose tissue
- insulin sensitivity
- Anti-inflammatory effect
- fatty acid uptake and beta-oxidation and muscle and liver
- fatty acid synthesis and gluconeogenesis
What is peroxisome proliferator-activated receptors (PPARs) and their function?
Transcriptional factor that responds to dietary lipids
- Promotes fat storage in adipose tissue
- Promotes fatty acid oxidation in muscle and liver
- Promotes adipocyte differentiation from pre-adipocyte
- Acts by forming heterodimers with another transcripti9nal factor RXR
How do the 3 types of PPARs work?

What factors released by adipocyte affect peripheral tissues and how?
- Resistin - increase insulin resistance
- TNF(alpha) - increase insulin resistance
- Free fatty acids - increase insulin resistance
What are the effects, molecularly, of fat accumulation?
- Dysregulation of adipokine production (i.e. leptin)

What are the effects of obesity on fat storage and what are its consequences?
- Adipocytes are filled with TAGs so no more can be stored in adipose tissue to meet demand
- Adipocytes are insulin resistant
- Decreased expression of genes promoting adipocyte development (i.e. PPAR)
- Increased expression of genes promoting adipocyte development in muscle and liver
- fat storage in muscle and liver
- Excess TAG in muscle and liver = TOXIC
What is the endocrine signalling from adipose tissue mechanism in obesity?
- Insulin resistance –> + glucose output from liver
- glucose uptake by muscles
- adipose
- blood glucose
How does an increase in free fatty acids lead to insulin resistance?
- Lead to inflammation –> + inflammatory cytokines
- Inflammatory cytokines inhibit insulin signalling
What are the basic principles of type 2 diabetes?
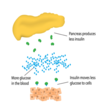
What are the steps leading to type 2 diabetes?
- Increase in insulin resistance
- fatty acids
- Beta cell compensation - hyperinsulinemia
- Beta cells cannot meet insulin demand due to + glucose output and insulin resistance
- Impaired glucose tolerance and + glucose in blood
- Decreased insulin secretion due to insulin resistance
- Diabetes

What are the HBA1c values for pre-diabetics?
42-47 Mmol/mol
What things put people at higher risk of developing type 2 diabetes?
- Relative with it
- Ethnicity
- Obesity
- Previous IGT, IFG or gestational diabetes
- Dyslipidaemia
- Sedentary lifestyle
- Smoker
- Small birth weight
What is the patholhysiology of type 2 diabetes?
- Insulin resistance –> ageing, genetics, obesity
- Environmental factors –> Improper diet, sedentary lifestyle
- + fatty acids –> + lipolysis in visceral fat
- Increased glucose output –> + gluconeogenesis
- Hyperinsulinemia –> B-cell compensation
- - insulin secretion
- B-cell decompensation
- Impaired glucose tolerance
- Type 2 diabetes
What is the diabetes prevention programme for?
Prevent or delay development of type 2 diabetes in someone with IGT
How is Metformin used?
850mg twice daily
What are the pharmacological strategies to prevent type 2 diabetes?
- Reduce endogenous liver glucose production
- Stimulate insulin secretion
- Reduce insulin resistance
- Reduce gut absorption of glucose
- Suppress appetite
- Delay gastric emptying
- Increase urinary glucose secretion
What are the functions of Metformin?
- Reduce endogenous liver glucose production
- Reduce insulin resistance
What is the function of sulphonylureas?
Stimulate insulin secretion
What is the function of DPP4 inhibitors?
Stimulate insulin secretion
What are the functions of GLP-1 analogues?
- Stimulate insulin secretion
- Suppress appetite
- Delay gastric emptying
What is the function of glitazones?
Reduce insulin resistance
What is the function of alpha glucosidase inhibitors?
Reduce gut absorption of glucose
What is the function of SGLT2 inhibitors?
Increase urinary glucose excretion
What is the mechanism of metformin?
Inhibit electron complex in mitochondria
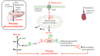
What are the positives of Metformin?
- Inexpensive
- Weight neutral
- Effective glucose lowering
- Probably cardio-protective
- Possibly cancer-protective
What are the negatives of Metformin?
- Not tolerated in up to 20% of patients
- eGFR < 30 ml/min contraindicated
What are the positives of sulphonylureas?
- Cheap
- Lowers glucose
What are the negatives of sulphonylureas?
- Weight gain
- Hypoglycaemia
- CVD risk ?
How do sulphonylureas work?
+ insulin secretion

What are the positives of alpha glucosidase inhibitors?
- Lowers glucose
- No hypoglycaemia
- CVD risk lowered ?
What are the negatives of alpha glucosidase inhibitors?
- Poorly tolerated (GI side effects)
- Relatively weak
- Avoid if GFR < 30 ml/min
How do alpha glucosidase inhibitors work?

What are the positives of PPAR gamma activator?
- Lowers glucose
- Improved CVD risk
- No hypoglycaemia
- Ok in renal disease
What are the negatives of PPAR gamma activator?
- Weight gain (5-7 kg)
- Fluid retention
- Heart faikure
- Anaemia
- Increased bone fractures
- Increased bladder cancer risk
How does GLP-1 work on pancreas?
Alpha-cells:
- Reduces post-meal glucagon secretion
Beta-cells:
- Increases glucose-dependent insulin secretion
How does GLP-1 work on liver?
Increased insulin and reduced glucagon in portal vein reduces hepatic glucose output
How does GLP-1 work on stomach?
Slows gastric emptying
How does GLP-1 work on hypothalamus?
Promotes satiety and reduces appetite
What is the overall result of GLP-1 use?
Lowers blood sugar and weight loss overtime


