(gastro) gut immunology Flashcards
what is the surface area of the GI tract?
approx 200 square metres
explain why the GI tract is said to have a massive antigen load
resident microbiota = 10^14 bacteria
dietary antigens
exposure to pathogens
= collectively result in an a huuuge antigen load
what is microbiota?
mixture of microorganisms that make up a community in an environmental niche
what is the microbiome?
collective genomes of all the microbiota
differentiate between microbiota and microbiome
microbiota = mixture of microorganisms that make up a community in an environmental niche
microbiome = collective genomes of all the microbiota
explain why the GI tract is in a state of ‘restrained activation’
has to balance tolerance of food antigens and commensal bacteria with the active immune response against foreign pathogens

why is bacterial microbiota important?
for the immune homeostasis of the gut and the development of a healthy immune system
how much gut bacteria is present in our body?
approx 10^14 gut bacteria
what makes up the gut microbiota?
four major phyla of bacteria (Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria), also viruses & fungi
what does the gut microbiota provide?
provide traits we have not had to evolve on our own
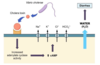
how is bacterial growth stimulated by the host?
via ingested nutrients or secreted nutrients
how is bacterial lysis/elimination stimulated by the host?
via chemical digestive factors
OR
via peristaltic contractions and defecation
which host actions lead to an increase in cell numbers?
ingested nutrients or secreted nutrients
= bacterial growth
= increased bacterial cell numbers

which host actions lead to a decrease in cell numbers?
chemical, digestive factors + peristaltic contractions + defecation
= bacterial lysis and elimination
= decreased bacterial cell numbers

why are microbiota important to the host?
provide essential nutrients
metabolise indigested components
defence against colonisation by opportunistic pathogens,
contribute to intestinal architecture
what is the effect of ingested/secreted nutrients in the gut microbiota?
stimulate bacterial growth
= increased bacterial cell numbers

what is the effect of chemical digestive factors in the gut microbiota?
stimulate bacterial lysis
= decreased bacterial cell numbers

what is the effect of peristalsis, contractions and defecation in the gut microbiota?
stimulate bacterial elimination
= decreased bacterial cell numbers

what chemical, digestive factors are produced by the host in the stomach, and what is the impact of this?
hydrochloric acid (pH 1-4)
pepsin
gastric lipase
= chemical, digestive factors that stimulate bacterial lysis and therefore decrease bacterial cell numbers
(10^1)

what chemical, digestive factors are produced by the host in the liver and what is the impact of this?
bile acids
= stimulate bacterial growth and therefore result in increased bacterial cell numbers
(10^3)

what chemical, digestive factors are produced by the host in the pancreas, and what is the impact of this?
trypsin
amylase
carboxypeptidase
= secreted nutrients that stimulate bacterial growth and therefore increase bacterial cell number
(10^4)

what chemical, digestive factors are produced by the host in the small intestine, and what is the impact of this?
brush border enzymes
= increase bacterial content
(10^7)

what chemical, digestive factors are produced by the host in the colon, and what is the impact of this?
no host digestive factors
= reduced bacterial lysis
= bacterial cell numbers cannot remain decreased
= huuuge increase in colonic bacteria
(10^12)

define symbiosis
any relationship or interaction between two dissimilar organisms (not always to the mutual benefit of either species)
the specific kind of symbiosis depends on whether either or both organisms benefit from the relationship