Drug effects on the hypothalamic pituitary axis Flashcards
Describe the pathway for GH release and its effects?
GH released in response to GHRH and ghrelin from brain
Release inhibited by somatostain
Gh released from pituitary and travels to liver
Liver is stimulated to produce IGF-1 > effects of GH
IGF-1 provides negative feedback to pituitary

Which parts of the GH pathway can go wrong?
Hormone insensitivity: can make a hormone that doesn’t work
End up with high levels of GH
Secondary deficiency: pituitary doesn’t produce growth hormone
Tertiary deficiency: no release of GH, because brain isn’t telling it to be released
How can GH be administered? Why?
How often must it be administered? Why?
Parenteral administration only, as it has zero bioavailability after oral administration
Administered daily or multi-daily, as it has a short half-life in the plasma
What does of GH is administered to patients?
Titrate dose to effect
Varies across patients
Describe the effect of Gh on T4?
GH can cause reduced T4 levels
What is somatotropin?
GH
Describe the effect of modifying ghrelin on GH?
Get more GH release for any given level of GHRH in the presence of more ghrelin

In which cases is treatment with IGF-1 useful?
GH insensitivity
Patients with anti-GH Ab
Describe the side effects of treatment with IGF-1?
Like insulin > acute hypoglycaemia
Describe the effects of having too much growth hormone?
Depending on when you have too much, can become very large (gigantism) or parts of your body can become very large (acromegaly)
Describe the approach to treatment of excess GH?
Remove tumour
Reduce GH release
Inhibit GH action
How can tumours that are causing excess GH release be localised?
Use receptor kinetics to localise
Somatostatin receptors internalise upon activation, taking peptide ligand with them
Tumours expressing somatostatin receptors can be imaged by in vivo receptor scintigraphy
Describe the approaches to reducing GH release?
Somatostatin administration
Somatostatin reduces GH release from pituitary
Describe the effect of somatostatin administration?
Reduced GH release from pituitary
How is somatostatin administered?
Parenteral administration only
Short-half life > regular injections
In which form is somatostatin administered?
Why?
Octreotide and lanreotide
Somatostain analogues that have been modified to prevent enzymatic cleavage and extend half-life (somatostain has a very short half-life)

How is resistance to enzymatic cleavage commonly achieved?
Insert ‘unnatural’ D amino acids

How can the effectiveness of treatment with somatostatin analogues be increased?
In some patients, addition of dopamine agonists
How can GH action be inhibited?
Administration of GH antagonists
How can GH receptor binding be disrupted?
Alter glycine at position 120 (addition of side chain in receptor prevents site 2 binding)
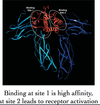
Describe the pharmacokinetic properties of G120K-GH?
High affinity antagonist
Short half-life
How is the half-life of G120K-GH increased?
PEGylation
Addition of PEG:
increases size, which reduces renal filtration
improves solubility
decreases access for proteolytic enzymes

What is the problem with PEGylated G120K-GH?
How was this overcome?
PEGylation occurs at lysines, but there are two lysines involved in site 1 binding
So, end up with completely inactive molecule
Further mutated molecule to produce pegmisovant
Describe the pathway for thyroid hormone release?
TRH > pituitary > TSH > thyroid gland > thyroid hormone
Thyroid hormone provides negative feedback to both pituitary and brain







