Cycle 3 - Thermodynamics and Membranes Flashcards
How does the second law apply to living systems?
- Cells are islands of low entropy.
- Life is endergonic; it constantly needs inputs of energy.
- It uses energy to build things, like testosterone and fatty acids.
- But why do we need to keep making energy? Everything in the cell breaks down (sugars, proteins, membranes). It is a constant cycle of building things up and breaking them down; of fighting entropy
State the equation for free energy
How is it influenced by enzymes?
- Reactions that are exergonic (i.e. negative G) are spontaneous.
- Spontaneous means the reaction will proceed as written on its own (no input of energy)
- Enzymes can speed up exergonic reactions by lowering the activation energy
- Enzymes cannot speed up endergonic reactions

Compare thermodynamics and kinetics
- Thermodynamics is net change in energy - due to the nature of the reaction
- Kinetics is the path of the reaction - affected by enzymes
What is required for a protein to fold correctly and what is it that holds the required information?
- Protein folding is spontaneous; it happens by itself
- If it doesn’t fold properly, it probably is not function –> especially for enzymes, where shape is important for function
- The folding is simply dictated by its sequence
- A protein with a different ordering of amino acids will fold differently, etc.
How is urea used to unfold proteins?
- Urea is used to denature proteins; unfold them
- It is effective because it competes with hydrogen bonds, taking their place and thus preventing bonding
- Disrupts tertiary and secondary sequences
State the basics of enzyme structure and the catalytic cycle.
- Enzymes are flexible and fit into substrates by induced fitting
- Catalysis takes place at the active site
- Enzyme E + substrate S = ES enzyme-substrate complex = free enzyme E + free produce P
- E + S = ES = E + P
- This is the catalytic cycle
State the four levels of protein structure and their binding arrangements
- Primary structure is the sequence of amino acids forming a polypeptide
- Secondary structure is produced by the twists and turns of the amino acid chain
- Tertiary structure is the folding of the hydrophobic amino acid chain into the overall three-dimensional shape of a protein. Bonds using R-groups.
- (optional) Quaternary structure refers to the arrangement of polypeptide chains in a protein that is formed from more than one chain.

State the role of size and charge in movement of molecules across a membrane
Certain things sneak through the membrane, such as O2 or CO2. They go through membranes because they are small and uncharged (non polar).
Very small uncharged polar molecules (water) also move through the membrane, but bigger charged molecules (glucose) can’t
What happens when a membrane is too fluid?
Membranes can’t be too fluid or else ions sneak through. Likewise, if it’s too viscous things don’t move fast enough
Compare transmembrane and peripheral proteins
- Transmembrane protein: proteins that are embedded in the phospholipid bilayer
- Because they have to interact with both the aqueous environment on either side of the membrane and the hydrophobic core, transmembrane proteins have two distinct parts (called domains) that differ in polarity (exposed = polar, internal = nonpolar)
- Transmembrane proteins can be identified by the presence of stretches of amino acids that are primarily non-polar.
- Peripheral protein: proteins held to membrane surfaces by noncovalent bonds formed with the polar parts of integral membrane proteins or membrane lipids.
Describe simple and facilitated diffusion
- Simple diffusion: mechanism by which certain small substances diffuse through the lipid part of a biological membrane.
- Facilitated diffusion: mechanism by which polar and charged molecules diffuse across membranes with the help of transport proteins.
Describe transport against a concentration gradient (active transport)
Active transport: the mechanism by which ions and molecules move against the concentration gradient across a membrane, from the side with the lower concentration to the side with the higher concentration.
- ATP is required because the transportation would cause a decrease in the entropy of the system
What drives transport?
- Transport is driven from change in energy from low to high
- The molecules want to spread out to areas of low concentration (spontaneous)
What is the ABC transporter?
Consists of an ATP-binding domain (not specific to the molecule) and a transmembrane domain (highly specific to the molecule)
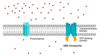
Explain cystic fibrosis, CFTR, delta F508, chaperone proteins, the ER quality control system, and proteasomes
- A famous example of an ABC transporter is CFTR, which lines the lungs, intestines, pumps chloride, and is important for keeping the epithelial lining wet to diffuse oxygen
- Cystic fibrosis is caused by a mutation to the CFTR that causes the protein to fold incorrectly
- The most common mutation is the removal of F508, a protein folding mechanism
- The HSP90 chaperone protein checks proteins in the ER such as the F508 and sends defective ones to recycling (“ER quality control system”)
- Here it is broken down by proteasomes
- However, if F508 CFTR is made in a lab and is put into the plasma membrane, it will pump chloride (not as well, but still not so bad) –> cure cystic fibrosis if we can deactivate HSP90?
How do you target a protein to the ER? i.e. how does it enter the secretory pathway?
- A protein that is destined for the secretory pathway has a targeting sequence called a signal peptide which is recognised by a signal-recognition particle which pulls it to the ER
- The enzyme in the lumen of the ER cleaves the signal peptide
- The signal peptide has no function in the mature protein
What are fatty acids?
State their role in membrane structure
Compare saturated vs. unsaturated
The fluidity of the lipid bilayer is influenced primarily by two factors: the type of fatty acids that make up the lipid molecules and the temperature.
The more C=C kinks in the fatty acid chain, the more fluid the membrane becomes
- Saturated: has no double bonds
- Unsaturated: has a double bond which creates a kink. Make the membrane more fluid, less viscous.
State the relationship of temperature on membrane fluidity
Also, state the relationship of bacterial desaturase expression vs. temperature.
- As the temperature drops and the random molecular motion of lipid molecules slows down, a point is reached where fluidity is lost and the phospholipid molecules form a semisolid gel
- Thus, cold = lots of desaturase
- High temperature = increase in fluid
- Thus, hot = low amount of desaturase
Explain the graph

- Enzymes become too rigid at low temperatures
- Thus, more enzymes must be synthesized to adapt
- Enzymes unfold at high temperatures, destroying active sites


