Chapter 5: Thermal Properties of Crystals Flashcards
Thermal Properties of Crystals
(3 points)
- Specific heat
- Thermal expansion
- Thermal conductivity
Specific Heat
The amount of energy needed to raise the temperature of a substance by one degree
First law of Thermodynamics
dQ = dU - dW = dU + pdV
Specific Heat at Constant Pressure and Volume
(3 points)
- CP - CV = TVαV2B
- αV ≡ thermal expansion coefficient
- B ≡ bulk elastic modulus

Specific Heat: Classical Treatment
(Assumptions: 2 points)
- 3r’N independent vibration modes
- Equipartition theorem states each mode contant kBT/2 potential and kinetic energy each

Specific Heat: Classical Treatment
(Take-Away: 3 points)
- Dulong-Petit specific heat
- CV = 3r’NkB
- Only valid for high T
Specific Heat: Quantum Treatment
(Assumptions: 2 points)
- 3N harmonic oscillators
- Temperature T
Specific Heat: Quantum Treatment
(Take-Away: 6 points)
- Expectation value of internal energy (see below)
- For r-atom basis
- 3N → 3rN
- ω → ωqr
- < n > → < nqr >
- <U > → (see below)

Specific Heat: Einstein Approximation
(Assumptions)
- All modes vibrate with same frequency ωE
Specific Heat: Einstein Approximation
(Take-Away: 3 points)
- ΘE = (h-bar)ωE/kB → Einstein temperature
- Works well for 200 K < T < 1300 K
- Good approximation when optical branches dominate
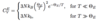
Specific Heat: Debye Approximation
(Assumptions: 7 points)
- All 3 phonon branches have linear dispersion ωi = viq
- Integrating over first Brillouin Zone equivalent to integrating overr sphere of radius qD
- Each q-state occupies volume (2π/L)3
- Each branch has N states
- qD = (6π2 N/V)1/3
- Debye frequency ωD = viqD
- Density of states in frequency space Z(ω) = (V/2π2vi2) ω2

Specific Heat: Debye Approximation
(Take-Away: 5 points)
-
ΘD = (h-bar)ωD/kB → Debye temperature
- Defines limit between classical and quantum treatment
- Measure of maximum phonon frequency
-
CV ∝ T3
- Agrees with experiment for low T
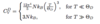
Specific Heat Comparison Graph

Number of Phonons
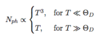
Anharmonic Effects
(Harmonic Short-Comings: 4 points)
- No thermal expansion
- Elastic constant independent of P,T
- CP = CV with CP constant for T > ΘD
- No phonon interactions
Anharmonic Potential
(Assumptions)
- Comes from considering more terms of Taylor expansion

Anharmonic Potential
(Take-Away: 4 points)
- Anharmonic coupling (3 phonon process)
- Two phonons create another one
- One phonon decays into two phonons
- Without anharmonic coupling, solid would never cool down
Anharmonic Potential: Conservation Laws
(4 points)
- Relaxed conservation laws (see below)
- Normal (n-)Process: G = 0; stays in first Brillouin Zone
-
Umklapp (u-)Process: Choose G so that q3 is in first Brillouin Zone
- Momenteum quasi-conserved, because some momentum transferred to lattice

Thermal Expansion
- Solids change length, volume when T is varied

Mean Particle Displacement
(3 points)
- For 1D oscillator:
- Harmonic Oscillator: < u > = 0
- Anharmonic Oscillator: < u > = 3b/(4a2)kBT**

Thermal Conductivity
(Overview: 5 points)
- Heat is transported by phonons and electrons
- Electronic constribution dominates in metals
- Crystalline insulators good thermal conductors at low T
- Non-equilibrium quantity
- Requires temperature gradient
Thermal Conductivity
(Definition)
- Expression for K can be derived from Kinetic Gas Theory

Kinetic Gas Theory
(Assumptions: 4 points)
- Phonons has of quasi-particles
- Average particle current in +x-direction jx = n< |vx| >/2
- Every particle delivers thermal energy ∆Q = C’V ∆T
- Mean free-path l
Kinetic Gas Theory
(Take-Away: 3 points)
- Obvious T dependence
- CV = CV(T)
- l = l(T)

Temperature-Dependence of K: Scattering Processes
(Dominant Scattering Processes: 2 points)
- phonon-phonon scattering
- defect, surface scattering
Matthiessen’s Rule
In case of several, independent scattering mechanisms, total scattering time τ can be determined by

Phonon-Phonon Scattering
(2 points)
- n-processes: Do not contribute to heat resistance
- u-processes: Loss of phonon momentum leads to heat resistance

Defect Scattering Contribution to K
(2 points)
- Probability proportional to defect density nD and scattering cross-section σ
- Contribution to K is independent of T
Temperature Dependence of K Results



