C2.1 Structure and Bonding Flashcards
Definition of a compound
Two or more elements which are chemically combined
Definition of Covalent Bonding
Shared pair of electrons
Definition of Ionic Bonding
(electrostatic) attraction between oppositely charged ions
Describe the structure of NaCl
Giant ionic lattic (structure)

Formula of Magnesium Iodide
MgI2
Type of bonding in graphite
Covalent
Describe the structure of diamond
Giant covalent structure where each carbon has 4 bonds

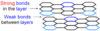
Describe the structure of graphite
Giant covalent structure where each carbon has 3 bonds. It is arranged in layers.
Definition of Metallic Bonding
(electrostatic) attraction between positive metal ions and the delocalised electrons

Type of bonding in CO2
Covalent
Describe the structure of CO2
Simple molecular
Identify the structure of H2O and describe its structure
Covalent
Simple molecular
Identify the type of bonding in copper and describe its structure
Metallic
Giant metallic lattice
Identify the type of bonding in sodium and describe its structure
Ionic
Giant ionic lattice
Identify the type of bonding in Ca(OH)2 and describe its structure
Ionic
Giant ionic lattice
Identify the type of bonding in SiO2 and describe its structure
Covalent
Giant covalent lattice/structure (giant molecular)
Graphite, diamond, nanotubes and fullerenes are all _________________ of carbon
allotropes
Formula of lithium sulphide
Li2S
Formula of a carbonate ion
CO32-
Formula of a sulphate ion
SO42-
Formula of a nitrate ion
NO3-
Formula of magnesium sulphate
MgSO4
Formula of calcium nitrate
Ca(NO3)2
Formula for methane and identify the bonding in methane
CH4
Covalent


