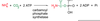Amino Acid Metab and Urea Cycle Flashcards
** concentration of free amino acids** is higher in the cell or out of the cell?
intracellular concentration is ocnsiderably higher
this gradient is maintained by active transport of amino acids into cells
the intracellular/extracellular free AA gradient varies with different AA, the greatest with
glutamate and glutamine.
** what are the most abundant amino acids in serum.
Glutamine and alanine
In the body there is about 100g of free amino acids.
how much is glutamate and glutamine?
how much is essential AA?
50% – glutamate, gluatmine
10% – essential AA
*********
What are the essenital AA?
“Pvt. Tim Hall”
Phenylalnine
Valine
Trptophan
Threonine
Isoleucine
Methionine
Histidine
Arginine
Leucine
Lysine
____ becomes essential if **methionine is low **
Cysteine
_____ becomes essential if phenylalanine is low.
tyrosine
essential AA for growth?
arginine
inputs into the AA pool are from
dietary protein and proteolysis of cellular protein
Approximately half the endogenous protein (6-7kg) is associated with
the skeleton and other supporting tissues
(Of which the major protein component is collagen)
Gastrointestinal inputs
70-100g of dietary protein and 35-200g of endogenous protein from sloshed off____ absorbed daily.
intestinal cells
Disorders associated with defects in amino acid transport lead to
increased levels of these amino acids in the urine,
Disorders associated with defects in amino acid transport
prognosis?
these disorders are benign or cause only minor health problems, since amino acids are also absorbed from the intestines as small peptides
___ separate transporters of AA from the gut into intestinal epithelial cells
FIVE
transport of amino acids from the gut into intestinal epithelial cell all are ___ symporters
NA or PROTON SYMPORTERS
High degree of redundancy with ___ since they overlap considerably in their specifity
AA transporters from gut to intestinal epithelial cells
what is the defect involved in Hartnup’s Disease??
A defect in the transport system for neutral and aromatic amino acids from the gut and the renal tubules
Hartnup’s Disease sx?
4D’s: Diarrhea, Dermatitis, Dementia, Death)
Hartnup’s Disease tx?
administration of niacin
how is Hartnup’s Disease dx?
diarrhea, dermatitis, dementia, death with HIGH LEVELS OF NEUTRAL OR AROMATIC AA IN URINE
what is the defect in Cysinuria?
A defect in the transport system for basic amino acids and cystine (a disulfide- linked dimer of cysteine) from the gut and the renal tubules.
Cystinuria sx?
crystals formed by cystine can lead to UTI AND KIDNEY STONES
Cystinuria tx?
fluids, and administration of the drug penicillamine (reacts with cystine to form a significantly more soluble compound)

where is trancytosis along the brush border most pronounced?
in infants where it enables them to acquire antibodies from breast milk.